Home > Products > intermediate
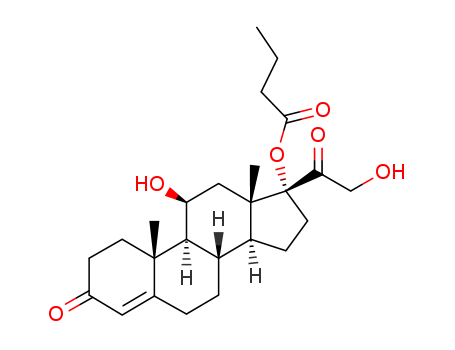







CasNo: 13609-67-1
MF: C25H36O6
|
Biochem/physiol Actions |
Hydrocortisone 17-butyrate is a corticosteroid that acts as a dermocorticoid due to its application in dermatological therapy. It exhibits therapeutic effects against vitiligo of the face and neck. |
|
Contact allergens |
Hydrocortisone 17-butyrate is a C 17 ester of hydrocorti- sone. It represents the D2 group of corticosteroids, non C 16 methylated with a C 17 ester: hydrocortisone 17-butyrate, hydrocortisone 17-valerate, hydrocortisone aceponate (17-propionate and 21-acetate), methylpred- nisolone aceponate, and prednicarbate. It is sometimes hydrolyzed in vivo into hydrocortisone, giving allergic reactions to group-A-sensitized people. |
|
Definition |
ChEBI: Cortisol esterified with butyric acid at the 17-hydroxy group. |
|
Brand name |
Locoid (Ferndale); Locoid (Yamanouchi). |
InChI:InChI=1/C25H36O6/c1-4-5-21(30)31-25(20(29)14-26)11-9-18-17-7-6-15-12-16(27)8-10-23(15,2)22(17)19(28)13-24(18,25)3/h12,17-19,22,26,28H,4-11,13-14H2,1-3H3/t17-,18?,19-,22?,23-,24-,25-/m0/s1
-
-
Composition for topical administration c...
17α-butyryloxy-11β-hydroxy-21-propionylo...
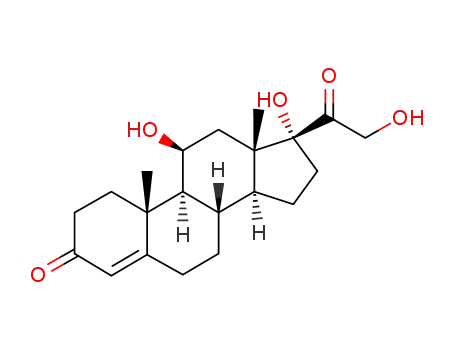
HYDROCORTISONE

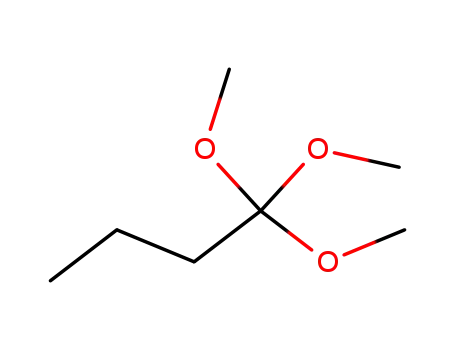
1,1,1-trimethoxybutane

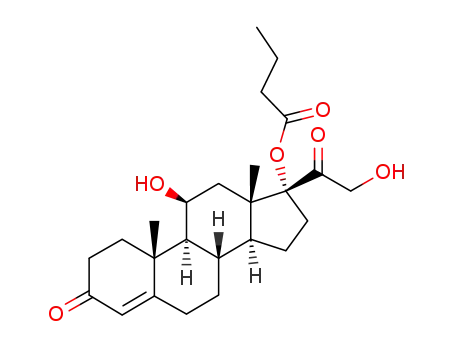
hydrocortisone 17α-butyrate

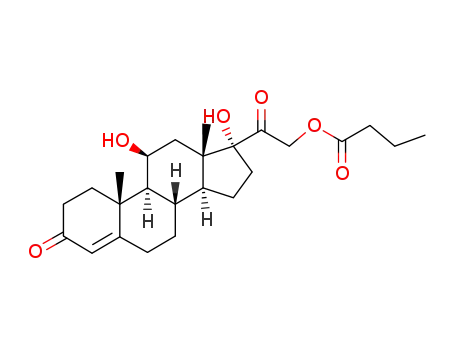
21-oxobutoxy-11β,17α-dihydroxypregn-4-ene-3,20-dione
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; toluene-4-sulfonic acid;
Yield given. Multistep reaction. Yields of byproduct given;
1) acetonitrile, 13 min, 2) acetonitrile, 14 min;
|
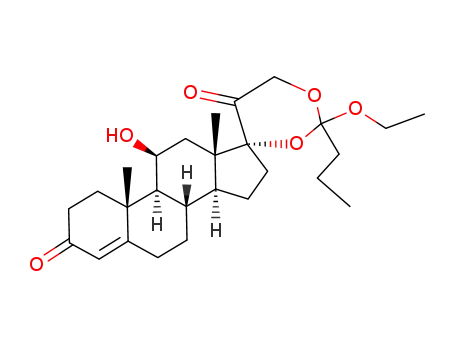
Hydrocortisone 17α,21-ethyl orthobutanoate


hydrocortisone 17α-butyrate
| Conditions | Yield |
|---|---|
|
aluminium silicate;
In
methanol; water;
for 1h;
Heating;
|
78% |

HYDROCORTISONE

1,1,1-trimethoxybutane

Hydrocortisone 17α,21-ethyl orthobutanoate
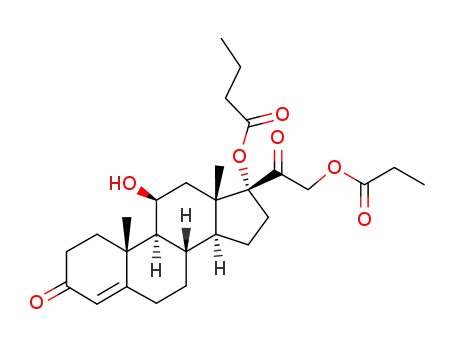
hydrocortisone butyrate propionate

HYDROCORTISONE

21-oxobutoxy-11β,17α-dihydroxypregn-4-ene-3,20-dione