Home > Products > intermediate
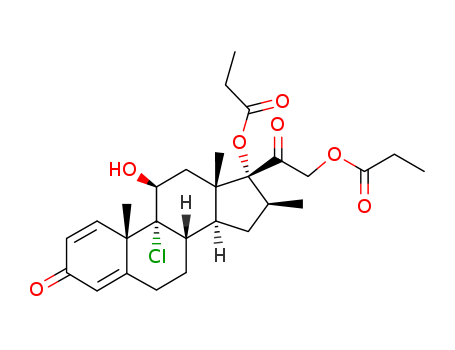







CasNo: 5534-09-8
MF: C28H37ClO7
Appearance: white crystalline powder
|
Biological Functions |
Beclomethasone dipropionate is used primarily as an inhalation aerosol therapy for asthma and rhinitis. A breakthrough in the discovery of new inhalation corticosteroids with reduced risks from systemic absorption was that the 17α-monopropionate ester of beclomethasone (17-BMP) was more active than BDP and 21-monopropionate (21-BMP) esters. Thus, BDP is a pro-drug that is rapidly metabolized by esterases in the lung and other tissues to its more active metabolite, 17-BMP, which has 30 times greater affinity for the GR than BDP and approximately 14 times dexamethasone (Table 33.5). |
|
Biological Activity |
beclomethasone dipropionate(bdp) is a topically active and anti-inflammatory corticosteroid used in treatment of asthma and rhinitis [1].beclomethasone dipropionate(bdp) is a topically active and anti-inflammatory corticosteroid used in treatment of asthma and rhinitis. in addition, beclomethasone dipropionate has been reported to be previously developed as aqueous nasal formulations for the treatment of allergic rhinitis. moreover, beclomethasone dipropionate has shown the availability in dry nasal aerosol formulations as chiorofluoro carbon metered-dose inhaler nasal sprays [1]. |
|
Side effects |
The main adverse effects are headache, sinusitis, and pain. Beclomethasone dipropionate is metabolized to the more active 17α-monopropionate derivative during absorption from the lungs and then further metabolized to the free alcohol in the liver. |
|
Metabolism |
The dipropionate also is metabolized to the inactive 21-monopropionate in the liver. Beclomethasone dipropionate and its metabolites are mainly excreted in the feces, with less than 10% excreted in the urine. |
|
references |
[1] ratner ph1, melchior a, dunbar sa, tantry sk, dorinsky pm. pharmacokinetic profile of beclomethasone dipropionate hydrofluoroalkane after intranasal administration versus oral inhalation in healthy subjects: results of a single-dose, randomized, open-label, 3-period crossover study. clin ther. 2012 jun;34(6):1422-31. |
|
Definition |
ChEBI: A steroid ester comprising beclomethasone having propionyl groups at the 17- and 21-positions. |
|
General Description |
Beclomethasonedipropionate (Beclovent, Beconase, Vanceril, Vancenase)(BDP) is rapidly converted in the lungs to beclomethasone17-monopropionate (17-BMP), the metabolite that providesthe bulk of the anti-inflammatory activity. The monopropionatealso has higher affinity for the GR than either thedipropionate or beclomethasone. The portion of BDP that isswallowed is rapidly hydrolyzed to 17-BMP, 21-BMP(which arises by a transesterification reaction from 17-BMP), and beclomethasone itself. Beclomethasone hasmuch less GC activity than the monopropionate. |
InChI:InChI=1/C28H37ClO7/c1-6-23(33)35-15-22(32)28(36-24(34)7-2)16(3)12-20-19-9-8-17-13-18(30)10-11-25(17,4)27(19,29)21(31)14-26(20,28)5/h10-11,13,16,19-21,31H,6-9,12,14-15H2,1-5H3/t16-,19-,20?,21-,25-,26-,27?,28-/m0/s1
Beclomethasone dipropionate (1) is a syn...
The invention provides a beclomethasone ...
The invention relates to a synthetic met...
The invention relates to a dipropionate ...
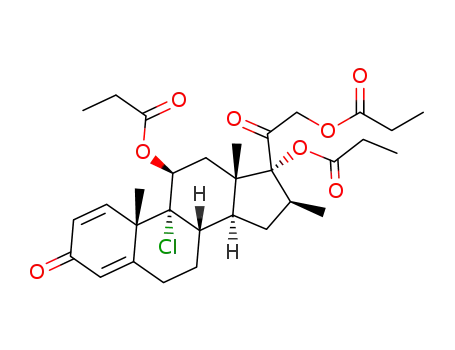
beclomethasone-11,17,21-tripropionate

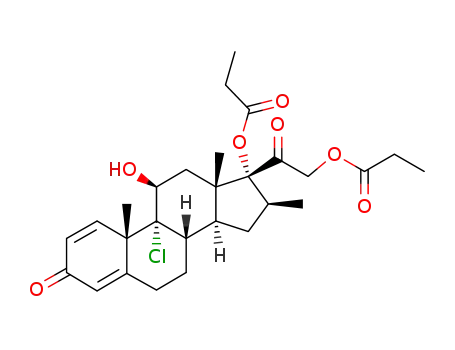
beclomethasone 17,21-dipropionate
| Conditions | Yield |
|---|---|
|
With
gluconic acid;
In
water;
at 0 ℃;
Reagent/catalyst;
Temperature;
|
99.2% |
|
With
gluconic acid;
In
tetrahydrofuran;
at 0 ℃;
for 1h;
Reagent/catalyst;
Solvent;
Temperature;
|
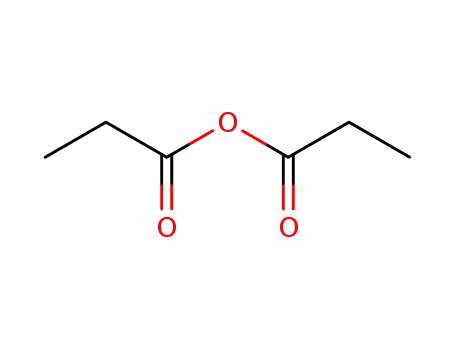
propionic acid anhydride

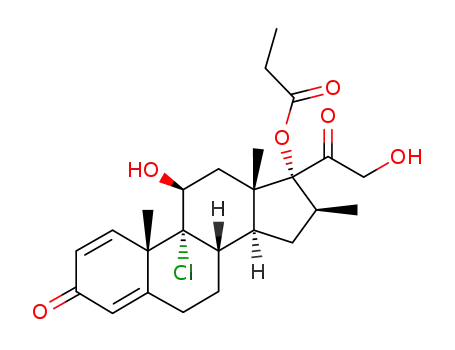
beclomethasone 17-monopropionate


beclomethasone 17,21-dipropionate
| Conditions | Yield |
|---|---|
|
With
dmap;
In
tetrahydrofuran;
at 20 ℃;
for 0.25h;
Inert atmosphere;
|
96% |

beclomethasone-11,17,21-tripropionate

propionic acid anhydride

beclomethasone 17-monopropionate
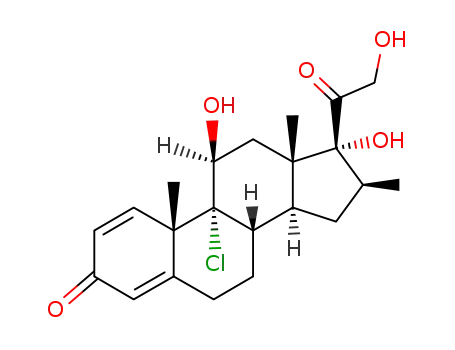
beclomethasone
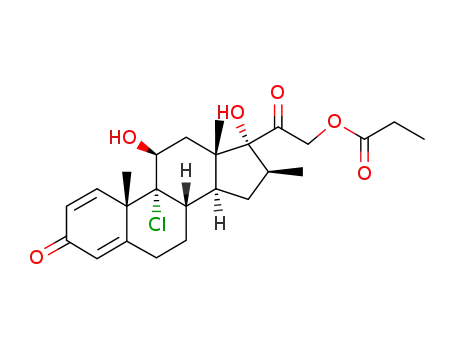
21-beclomethasone monopropionate

beclomethasone 17-monopropionate

beclomethasone
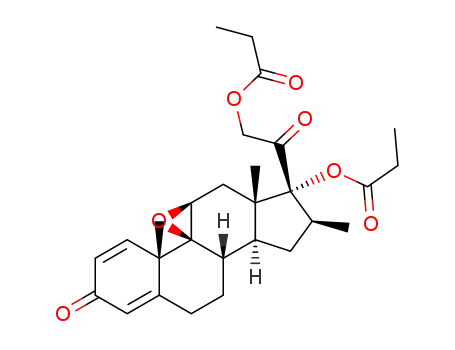
betamethasone 9,11-epoxide 17,21-dipropionate