Home > Products > intermediate







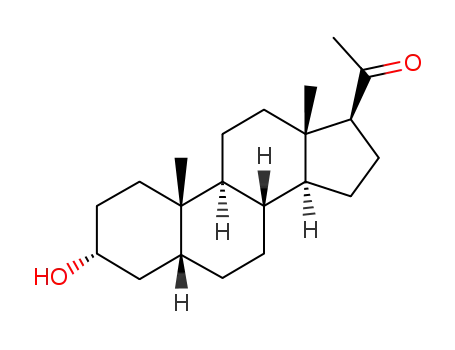
CasNo: 516-55-2
MF: C21H34 O2
|
Properties |
Mr of allopregnanolone is 318.49 and its melting point is 168°C. |
|
Discovery |
The presence of a progestational compound in the rabbit adrenal gland was reported in 1933, and was isolated in 1938 from the ox adrenal gland.Allopregnanolone formation was demonstrated in the brains of various vertebrates2–5 and the pineal glands of birds. |
|
Biological functions |
Allopregnanolone mediates its effects through modulation of the GABAA receptor.Allopregnanolone is reported to modulate the GABA-ergic function by increasing the GABAA receptor opening frequency and duration of the receptor at concentrations in the nanomolar range. A balance between unbinding, desensitization, and reopening of the desensitized GABAA receptor underlies the delay of the inhibitory postsynaptic currents.?Allopregnanolone slows the rate of recovery of the GABAA receptor from desensitization and possibly increases the rate of entry into fast desensitized states. Allopregnanolone exerts neurogenetic, neuroprotective, antidepressant, and anxiolytic effects. Reduced levels of allopregnanolone are found to be associated with major depression, anxiety disorders, premenstrual dysphoric disorder, and Alzheimer’s disease. Allopregnanolone is actively produced in the pineal gland compared with the brain and pineal allopregnanolone acts on Purkinje cells to prevent apoptosis in the juvenile quail. |
|
Clinical implications |
Decreased production of allopregnanolone leads to NP-C; thus allopregnanolone treatment may be useful in ameliorating progression of the disease.In patients with Alzheimer’s disease, the level of allopregnanolone in the temporal cortex was significantly lower than controls, in contrast to pregnenolone and dehydroepiandrosterone where the concentrations were increased.This may be explained by altered regulation of the?neurosteroid biosynthetic pathway, which blocks allopregnanolone formation. |
|
Regulation of synthesis and release |
Allopregnanolone synthesis is stimulated by swim stress.In swim stress models, increased allopregnanolone is associated with decreased dopamine and norepinephrine in the prefrontal cortex, suggesting that allopregnanolone influences the mesolimbocortical dopamine pathway.In vivo treatment of the rat with the dopamine D4 antagonist clozapine induces a rapid increase in the concentration of allopregnanolone in the cerebral cortex and striatum. |
InChI:InChI=1/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3/t14-,15+,16+,17-,18-,19-,20+,21-/m1/s1
-
-
20-Carboxamidopregnene derivatives, such...
Provided herein is a compound of Formula...
Predictability is a key requirement to e...
The present invention relates to a new p...
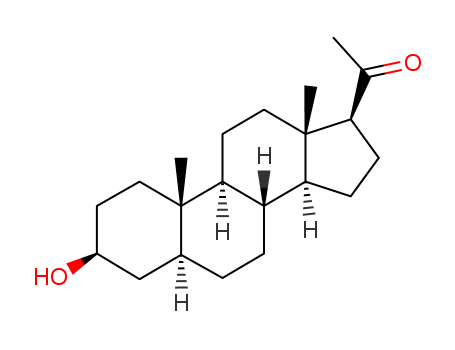
pregnanedione

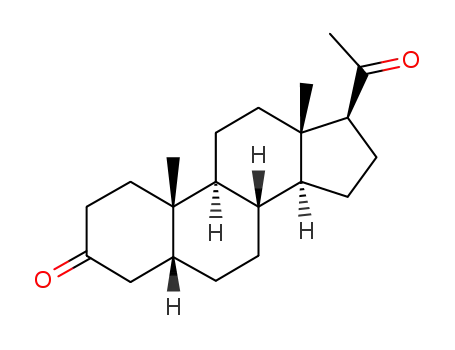
pregnadiol

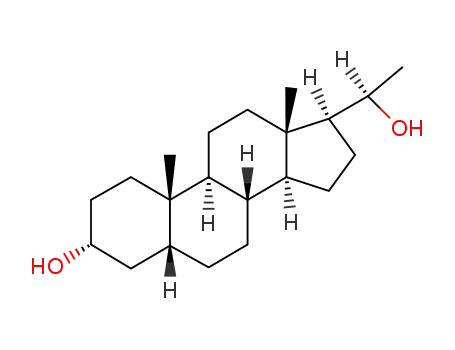
PREGNANOLONE
| Conditions | Yield |
|---|---|
|
With
recombinant human aldo-keto reductase 1C3; NADPH;
In
methanol;
at 37 ℃;
for 1.5h;
pH=7;
stereospecific reaction;
aq. phosphate buffer;
Enzymatic reaction;
|
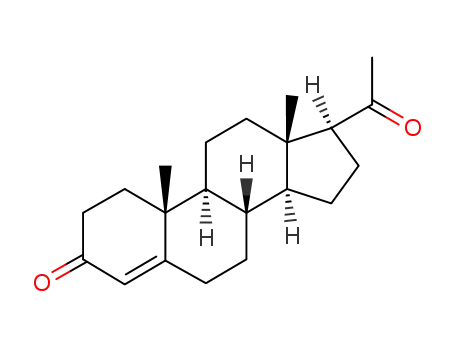
Progesterone


pregnanedione

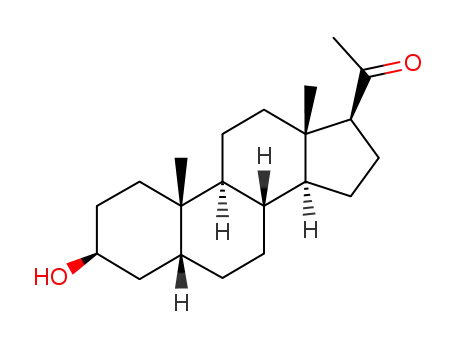
3β-hydroxy-5β-pregnan-20-one

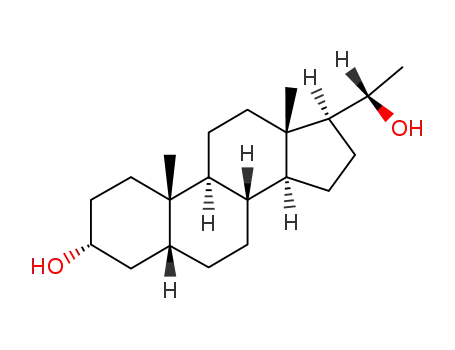
pregnanediol


pregnadiol


PREGNANOLONE

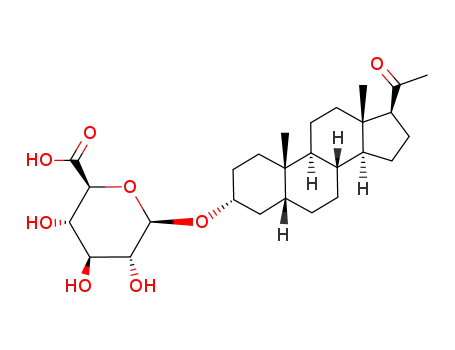
3α-hydroxy-5β-pregnane-20-one-3-glucuronide
| Conditions | Yield |
|---|---|
|
With
phosphate buffer; (S)-2-[3]pyridyl-pyrrolidine-1-carboxylic acid amide; α-D-glucose 6-phosphate; bovine liver tissue supernatant; NADP; UDP-glucuronic acid;
at 37 ℃;
for 5h;
Product distribution;
<14C>labeled;
|
4 % Chromat. 1 % Chromat. 22 % Chromat. 15 % Chromat. 46 % Chromat. |

Progesterone
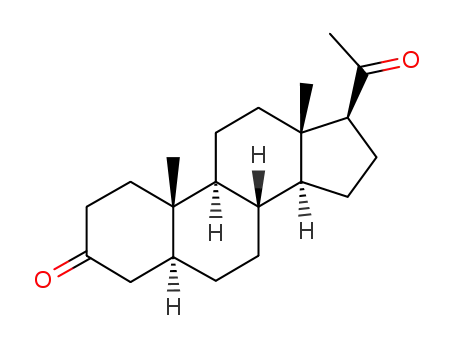
dihydroprogesterone
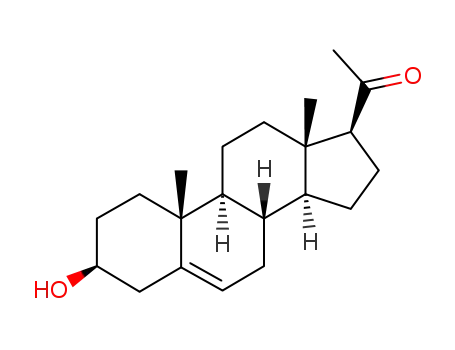
Pregnenolone
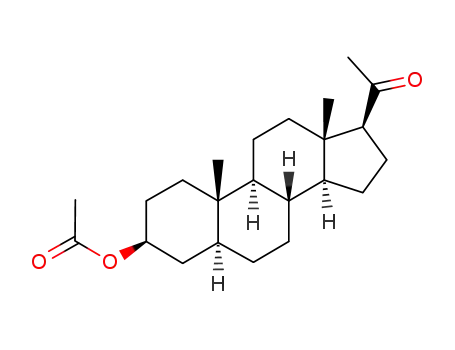
pregnenolone acetate
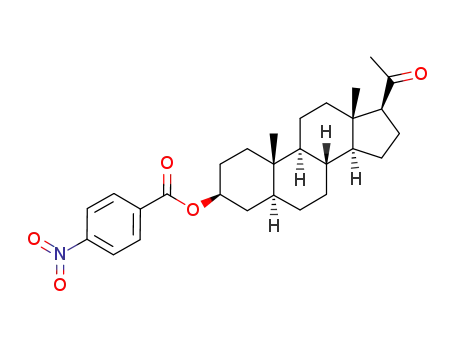
3β-(4-nitro-benzoyloxy)-5α-pregnanone-(20)
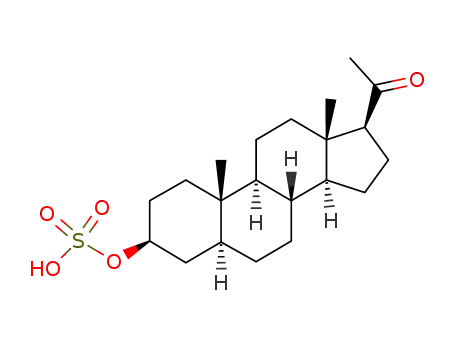
Isopregnanolone sulfate
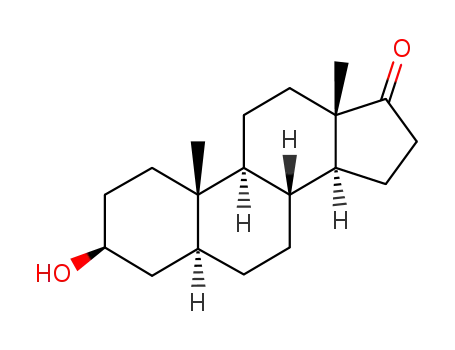
Epiandrosterone

dihydroprogesterone