Home > Products > intermediate
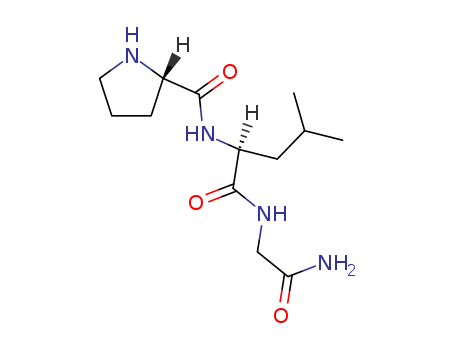







CasNo: 2002-44-0
MF: C13H24N4O3
|
Biological Activity |
melanocyte stimulating hormone release inhibiting factor,(c13h24n4o3), a tri-peptide with the sequence h2n-pro-leu-gly-amide, mw= 284.35. melanocyte-inhibiting factor (also known as pro-leu-gly-nh2, melanostatin, msh release-inhibiting hormone or mif-1) is an endogenous peptide fragment derived from cleavage of the hormone oxytocin, but having generally different actions in the body(1). mif-1 produces multiple effects, both blocking the effects of opioid receptor activation(2), while at the same time acting as a positive allosteric modulator of the d2 and d4 dopamine receptor subtypes, as well as inhibiting release of other neuropeptides such as alpha-msh, and potentiating melatonin activity(3). mif-1 is unusually resistant to metabolism in the bloodstream, and crosses the blood–brain barrier easily, though it is poorly active orally and is usually injected.figure1 formula of melanocyte stimulating hormone release inhibiting factor |
|
references |
1. Celis ME, Taleisnik S, Walter R (July 1971). "Regulation of formation and proposed structure of the factor inhibiting the release of melanocyte-stimulating hormone". Proceedings of the National Academy of Sciences of the United States of America 68 (7): 1428–33.2. Contreras PC, Takemori AE (June 1984). "Effect of prolyl-leucyl-glycinamide and alpha-melanocyte-stimulating hormone on levorphanol-induced analgesia, tolerance and dependence". Life Sciences 34 (26): 2559–66.3. Sandyk R (May 1990). "MIF-induced augmentation of melatonin functions: possible relevance to mechanisms of action of MIF-1 inmovement disorders". The International Journal of Neuroscience 52 (1-2): 59–65. |
InChI:InChI=1/C13H24N4O3/c1-8(2)6-10(12(19)16-7-11(14)18)17-13(20)9-4-3-5-15-9/h8-10,15H,3-7H2,1-2H3,(H2,14,18)(H,16,19)(H,17,20)/t9-,10-/m0/s1
A process for large scale liquid phase s...
The synthesis has been effected of the a...
-
-
PChd-L-Pro-L-Leu-Gly-NH2

2,6-di(t-butyl)-4-phenylphenol

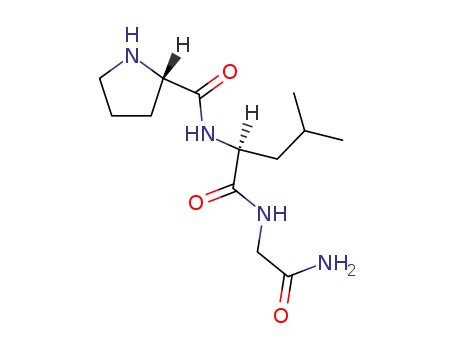
melanostatin
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium on activated charcoal;
In
methanol;
for 3h;
|
90% 85% |
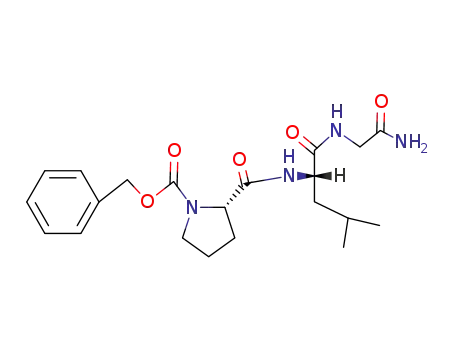
benzoyloxycarbonylprolyl-leucyl-glycine amide


melanostatin
| Conditions | Yield |
|---|---|
|
With
5%-palladium/activated carbon; hydrogen;
In
methanol;
|
99.7% |
|
With
hydrogen;
palladium;
In
ethanol;
|
80% |
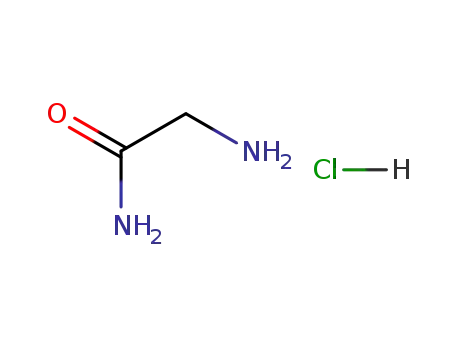
glycinamide hydrochloride
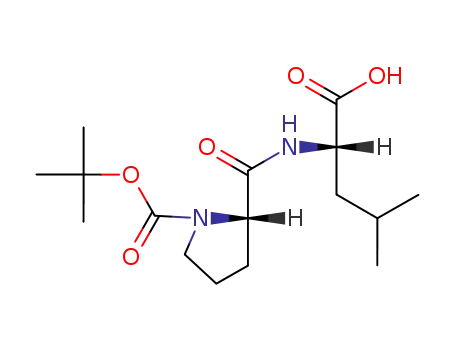
(2S)-2-{[(2S)-1-(tert-butoxycarbonyl)pyrrolidine-2-carbonyl]amino}-4-methylpentanoic acid
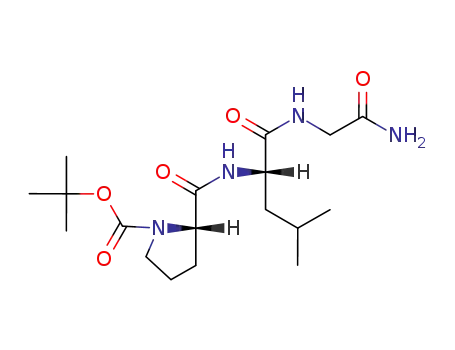
Boc-L-Pro-L-Leu-Gly-NH2
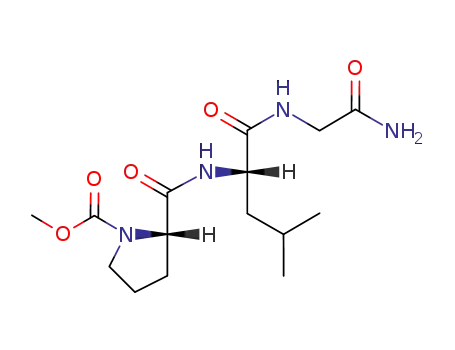
MOC-Pro-Leu-Gly-NH2
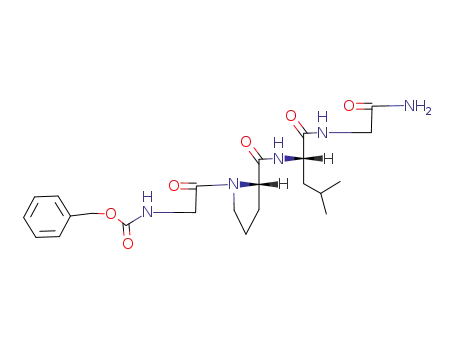
Benzyloxycarbonylglycyl-L-prolyl-L-leucyl-glycine Amide
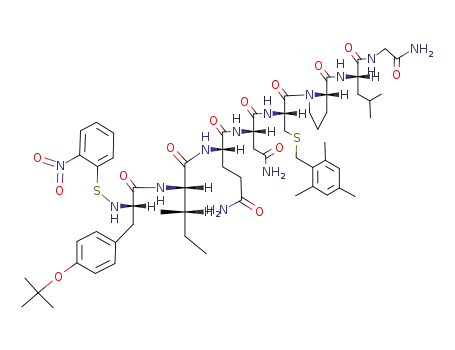
o-nitrobenzenesulfenyl-O-tert-butyl-L-tyrosyl-L-isoleucyl-L-glutaminyl-L-asparaginyl-S-(2,4,6-trimethylbenzyl)-L-cysteinyl-L-prolyl-L-leucyl-glycine amide
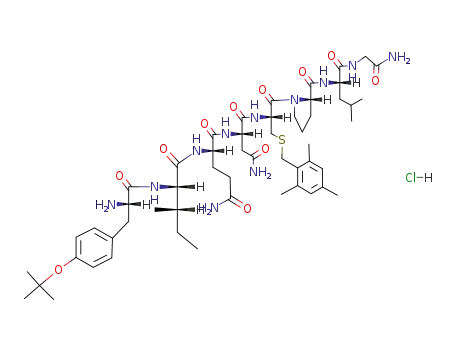
C54H83N11O11S*ClH
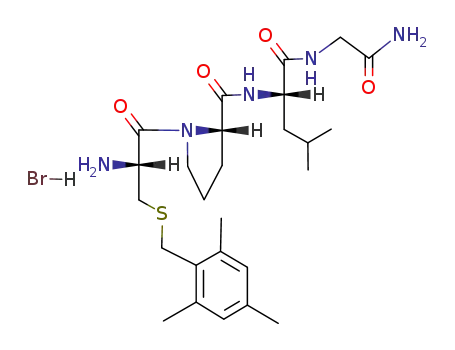
(S)-1-[(R)-2-Amino-3-(2,4,6-trimethyl-benzylsulfanyl)-propionyl]-pyrrolidine-2-carboxylic acid [(S)-1-(carbamoylmethyl-carbamoyl)-3-methyl-butyl]-amide; hydrobromide