Home > Products > intermediate
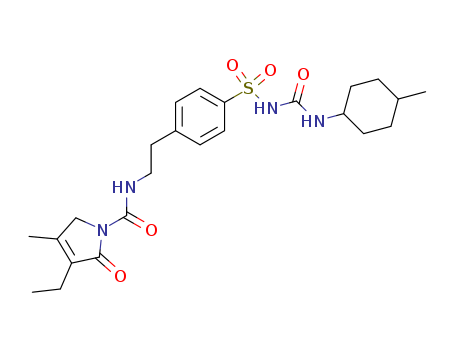







CasNo: 93479-97-1
MF: C24H34N4O5S
Appearance: white cyrstalline solid
|
Manufacturing Process |
By heating of a mixture of 3-ethyl-4-methyl-2-pyrrolone and 2- phenylethylisocyanate at 150°C is obtained 3-ethyl-4-methyl-2-oxo-3- pyrroline-1-(N-2-phenylethyl)-carboxamide, melting point 106°-108°C. Then the carboxamide are introduced in portions at 30°C into chlorosulfonic acid, and agitated for 1 hour at 40°C. The sulfochloride (melting point 172-175°C), introduced into concentrated ammonia, and heated for 30 min on a steam bath. The mixture of sulfonamide obtained (melting point 180°-182°C), of acetone and K2CO3 are refluxed with agitation for 6 hours. Subsequently the cyclohexyl isocyanate are added dropwise, and agitation is continued for 6 hours at boiling temperature. After standing overnight, the product is filtered, the crystals obtained are treated with dilute hydrochloric acid, and again filtered. It is prepared N-(4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1- carboxamido)ethyl]benzenesulfonyl)-N'-cyclohexyl urea; melting point 185°- 187°C (from acetone) (Glimepiride). |
|
Biological Activity |
Potent K ATP channel blocker and anti-diabetic agent. Inhibits pinacidil-activated cardiac K ATP channels with an IC 50 of 6.8 nM. |
|
Biochem/physiol Actions |
Glimepiride is a potent blocker of cardiac KATP channels activated by pinacidil with an IC50 of 6.8 nM. |
|
Veterinary Drugs and Treatments |
Glimepiride may potentially be a useful adjunct in the treatment of non-insulin dependent diabetes mellitus (NIDDM) in cats. Its duration of action in humans allows it to be dosed once daily, which could be of benefit in cats. It may also have fewer side effects than glipizide in cats. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Analgesics: effects enhanced by NSAIDs. Antibacterials: effects enhanced by chloramphenicol, sulphonamides, tetracyclines and trimethoprim; effect reduced by rifamycins. Anticoagulants: effect possibly enhanced by coumarins; also possibly changes to INR. Antifungals: concentration increased by fluconazole and miconazole and possibly voriconazole. Lipid-regulating drugs: possibly additive hypoglycaemic effect with fibrates. Sulfinpyrazone: enhanced effect of sulphonylureas. |
|
Metabolism |
The drug is extensively metabolised in the liver to two main metabolites. The cytochrome P450 isoenzyme CYP2C9 is involved in the formation of a hydroxy derivative, which is further metabolised to a carboxy derivative by cytosolic enzymes. About 60% of a dose is eliminated in the urine and 40% in the faeces. |
|
Classification and Structure |
Glimepiride is a third-generation sulfonylurea used in the treatment of type II diabetes mellitus. It shares a similar fundamental structure with glibenclamide and stimulates insulin secretion primarily by inhibiting the Sur1-regulated ATP-sensitive potassium channel. |
|
Pharmacokinetics and Pharmacodynamics |
Glimepiride exhibits high hypoglycemic efficacy and low systemic toxicity. It belongs to class II drugs according to the Biopharmaceutical Classification System (BCS), characterized by low solubility and high permeability. However, its poor water solubility leads to challenges in oral pharmaceutical preparation, dissolution profile, and bioavailability. |
|
Enhancement Techniques |
Various techniques are employed to enhance the solubility of poorly water-soluble drugs like glimepiride. These include prodrug formation, salt formation, crystal engineering, and solid dispersion methods using water-soluble polymers. Solid dispersion is particularly effective in improving aqueous solubility. |
|
Physical Properties |
Glimepiride does not undergo polymorphic transformations during processing, as indicated by studies using techniques like differential scanning calorimetry (DSC) and FT-IR spectroscopy. However, milling processes can induce changes in the hydrogen bonding patterns of glimepiride crystals. |
|
Mechanism of Action |
Glimepiride stimulates insulin release from beta cells in the pancreas and enhances the activity of intracellular insulin receptors. It undergoes slow gastrointestinal dissolution and exhibits inter-personal variations, affecting its bioavailability. |
|
Adverse Effects and Strategies |
Severe hypoglycemia can occur with large doses of glimepiride due to variations in absorption. Strategies such as inclusion complexes, solid dispersion, and micronization techniques have been employed to enhance dissolution, oral absorption, and bioavailability. Additionally, formulations like sublingual tablets and sustained-release tablets aim to improve drug absorption profiles and consistency. |
|
Brand name |
Amaryl (Sanofi Aventis). |
|
General Description |
Glimepiride is 3-ethyl-2,5-dihydro-4-methyl-N-[2-[4-[[[[(trans-4-methylcyclohexyl)amino]-carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo-1H-pyrrole-1-carboxamide; thiscompound can also be named as the urea—see precedingdiscussion (Amaryl, generic). Combinations are availablewith rosiglitazone in the United States (Avandaryl tablets;mg glimepiride/mg rosiglitazone as maleate salt: 1/4,2/4, 4/4, 2/8, 4/8); and with pioglitazone (Duetact tablets;mg glimepiride/ mg pioglitazone as hydrochloride salt:2/30, 4/30). |
InChI:InChI=1/C24H34N4O5S/c1-4-21-17(3)15-28(22(21)29)24(31)25-14-13-18-7-11-20(12-8-18)34(32,33)27-23(30)26-19-9-5-16(2)6-10-19/h7-8,11-12,16,19H,4-6,9-10,13-15H2,1-3H3,(H,25,31)(H2,26,27,30)/t16-,19?
The invention discloses a preparation me...
The invention discloses a process for sy...
The invention discloses a preparation me...
A novel and simple approach to the synth...
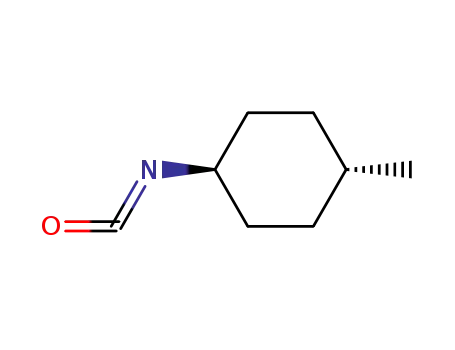
(1R,4R)-1-isocyanato-4-methylcyclohexane

![N-{2-[4-(aminosulfonyl)phenyl]ethyl}-3-ethyl-4-methyl-2-oxo-2,5-dihydro-1H-pyrrole-1-carboxamide](/upload/2025/4/5d1e027b-c073-4dce-a157-23f185ded47a.png)
N-{2-[4-(aminosulfonyl)phenyl]ethyl}-3-ethyl-4-methyl-2-oxo-2,5-dihydro-1H-pyrrole-1-carboxamide

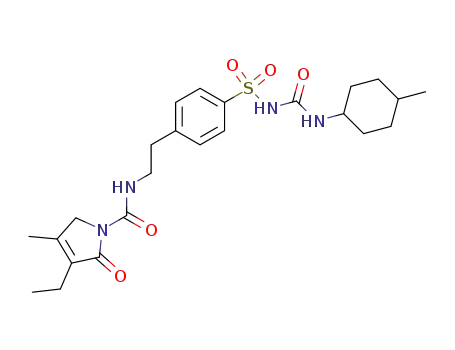
glimepiride
| Conditions | Yield |
|---|---|
|
(1R,4R)-1-isocyanato-4-methylcyclohexane; N-{2-[4-(aminosulfonyl)phenyl]ethyl}-3-ethyl-4-methyl-2-oxo-2,5-dihydro-1H-pyrrole-1-carboxamide;
With
potassium carbonate;
In
acetonitrile;
at 50 - 60 ℃;
for 6h;
With
water;
at 70 - 75 ℃;
for 4h;
|
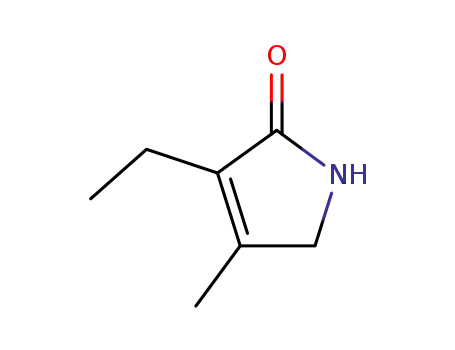
3-ethyl-4-methyl-3-pyrrolin-2-one


glimepiride
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1.1: toluene / 2 h / 120 - 130 °C / Industrial scale
2.1: chlorosulfonic acid / dichloromethane / -5 - 30 °C
3.1: ammonium hydroxide / methanol / 2 h / 20 - 30 °C / Industrial scale
4.1: potassium carbonate / acetonitrile / 6 h / 50 - 60 °C
4.2: 4 h / 70 - 75 °C
With
chlorosulfonic acid; ammonium hydroxide; potassium carbonate;
In
methanol; dichloromethane; toluene; acetonitrile;
|

(1R,4R)-1-isocyanato-4-methylcyclohexane
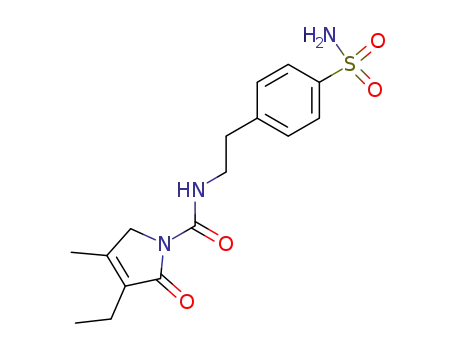
N-{2-[4-(aminosulfonyl)phenyl]ethyl}-3-ethyl-4-methyl-2-oxo-2,5-dihydro-1H-pyrrole-1-carboxamide

3-ethyl-4-methyl-3-pyrrolin-2-one
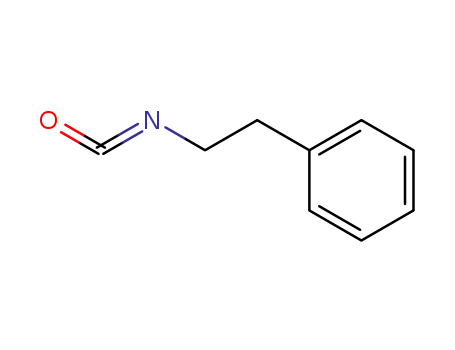
phenethyl isocyanate
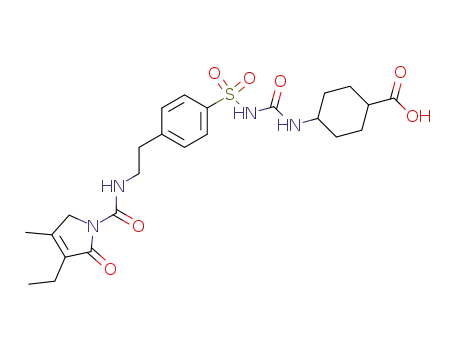
C24H32N4O7S
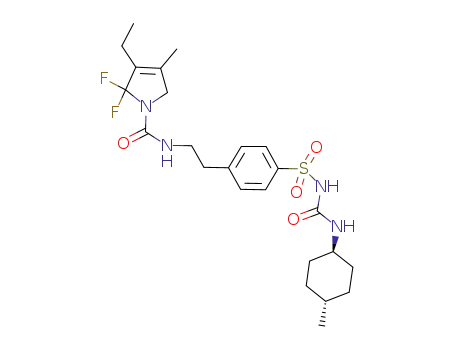
3-ethyl-2,5-dihydro-4-methyl-N-[2[4-[[[(trans-4-methylcyclohexyl)amino]carbonyl]aminosulfonyl]phenyl]ethyl]-2,2-difluoro-1H-pyrrole-1-N-carboxamide
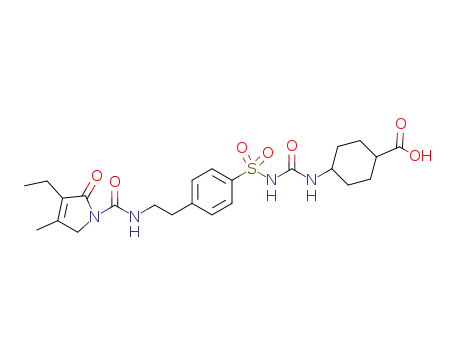
carboxyglimepiride
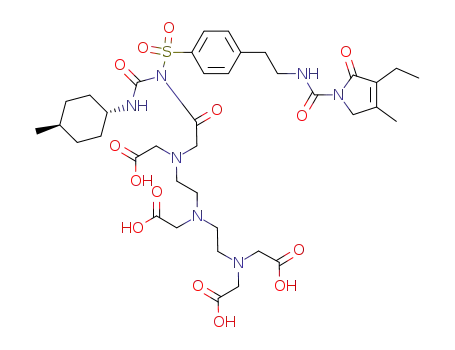
DTPA-glimepiride