Home > Products > intermediate
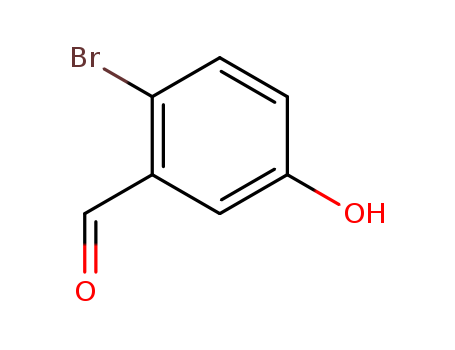







CasNo: 2973-80-0
MF: C7H5BrO2
InChI:InChI=1/C7H5BrO2/c8-7-2-1-6(10)3-5(7)4-9/h1-4,10H
Tricyclic cores of the daphnane diterpen...
The molecules of the title compound, C7H...
Synthesis of new Schiff's base Zn-comple...
Aryl-1,3-diones represent a promising ne...
-
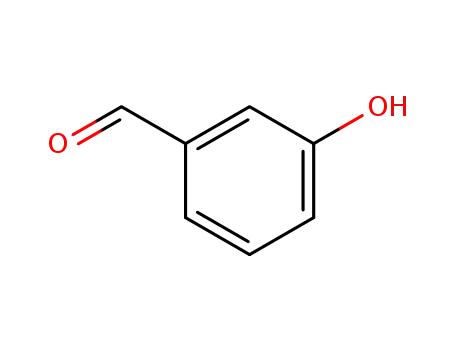
meta-hydroxybenzaldehyde

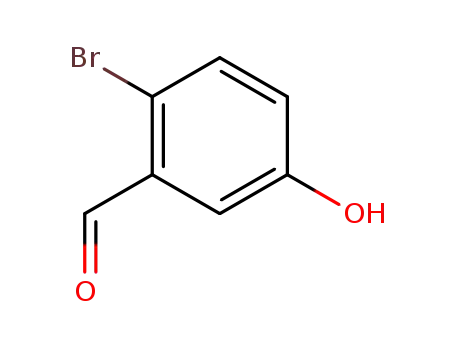
2-bromo-5-hydroxybenzaldehyde

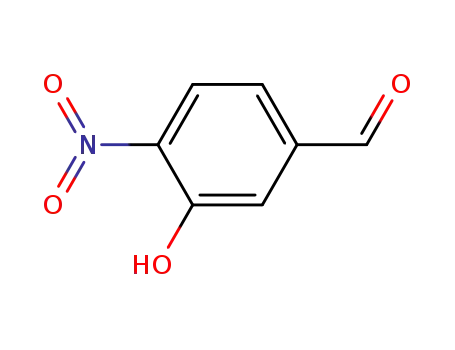
5-formyl-2-nitrophenol
| Conditions | Yield |
|---|---|
|
With
nitric acid; potassium bromide;
In
acetic anhydride;
at 20 ℃;
|
39 %Chromat. 35 %Chromat. |

meta-hydroxybenzaldehyde


2-bromo-5-hydroxybenzaldehyde
| Conditions | Yield |
|---|---|
|
With
bromine;
In
dichloromethane;
|
100% |
|
With
bromine;
In
dichloromethane;
|
100% |
|
With
1,3-di-n-butyl-1H-imidazol-3-ium tribromide;
at 20 ℃;
for 0.133333h;
regioselective reaction;
Neat (no solvent);
|
95% |
|
With
1-butyl-3-methylpyridinium tribromide;
at 20 ℃;
for 0.25h;
|
92% |
|
With
1,4-dioxane dibromide;
at 20 ℃;
for 0.5h;
|
80% |
|
With
bromine; iron;
In
acetic acid;
at 20 ℃;
|
80% |
|
With
bromine;
In
chloroform; acetonitrile;
for 3.5h;
|
78% |
|
With
bromine;
In
tetrachloromethane;
for 2.25h;
|
76% |
|
With
bromine;
In
dichloromethane;
at 0 - 2 ℃;
for 17h;
|
76% |
|
With
bromine;
In
dichloromethane;
at 0 - 20 ℃;
for 5h;
|
75% |
|
With
bromine;
In
dichloromethane;
at -5 - 25 ℃;
for 15h;
Inert atmosphere;
Large scale;
|
74% |
|
With
bromine;
In
chloroform;
for 3h;
Ambient temperature;
|
73% |
|
With
bromine;
In
acetic acid;
for 3h;
|
68% |
|
With
bromine;
In
dichloromethane;
Reflux;
Large scale;
|
65% |
|
With
bromine;
In
dichloromethane;
|
63% |
|
With
bromine;
In
dichloromethane;
at 35 - 40 ℃;
|
63% |
|
With
bromine; acetic acid;
|
57% |
|
With
bromine;
In
dichloromethane;
at 20 ℃;
for 1.5h;
Inert atmosphere;
|
56% |
|
With
bromine; acetic acid;
at 15 - 22 ℃;
|
55% |
|
With
bromine; acetic acid;
at 20 ℃;
|
55% |
|
With
bromine;
In
chloroform; acetonitrile;
at 20 ℃;
for 4h;
Cooling with ice;
|
55% |
|
With
bromine;
In
tetrachloromethane;
at 20 ℃;
for 2h;
|
51% |
|
With
bromine; sodium carbonate;
In
chloroform;
|
50% |
|
With
bromine;
In
dichloromethane;
at 25 ℃;
for 12h;
Inert atmosphere;
|
49% |
|
With
bromine;
In
dichloromethane;
for 3h;
Ambient temperature;
|
45% |
|
With
bromine; acetic acid;
at 10 - 20 ℃;
for 2.66667h;
Inert atmosphere;
|
35% |
|
durch Bromierung;
|
|
|
With
chloroform; bromine;
|
|
|
With
bromine; acetic acid;
|
|
|
With
bromine;
In
chloroform;
at 25 ℃;
|
|
|
Multi-step reaction with 2 steps
1: bei der Nitrierung
2: Na2S2O4; water / Diazotieren in bromwasserstoffsaurer Loesung und nachfolgende Behandlung mit Kupfer(I)-bromid
With
sodium dithionite; water;
|
|
|
With
bromine;
|
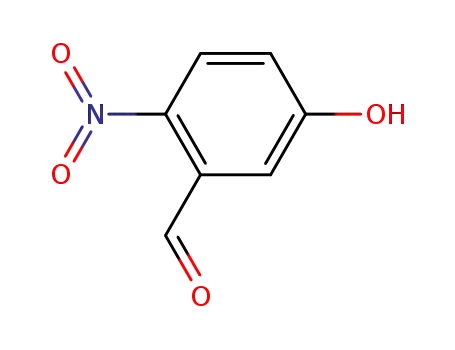
2-nitro-5-hydroxybenzaldehyde

meta-hydroxybenzaldehyde
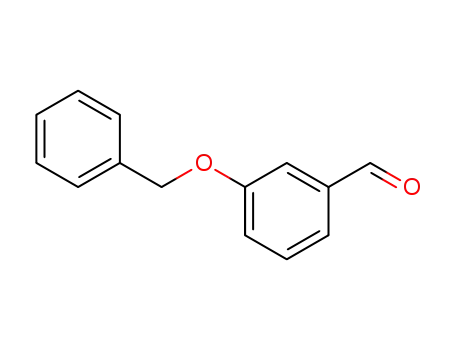
3-Benzyloxybenzaldehyde
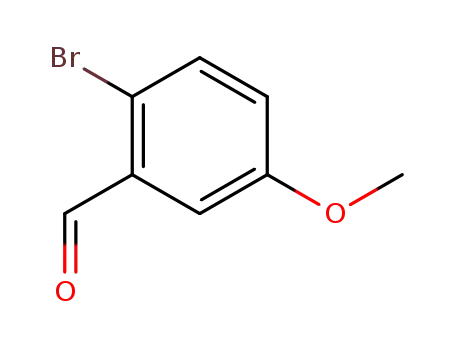
2-bromo-5-methoxy-benzaldehyde
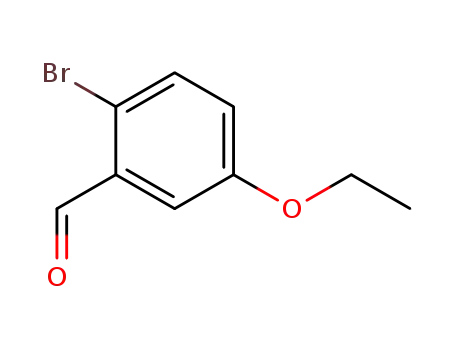
2-bromo-5-ethoxybenzaldehyde
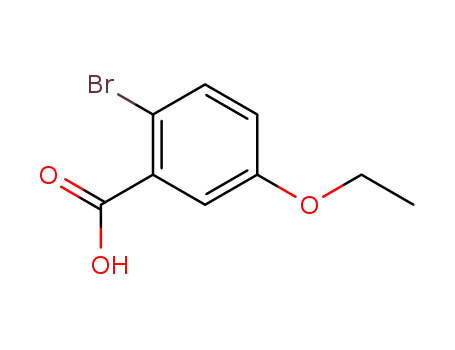
5-ethoxy-2-bromo-benzoic acid

2-bromo-5-methoxy-benzaldehyde
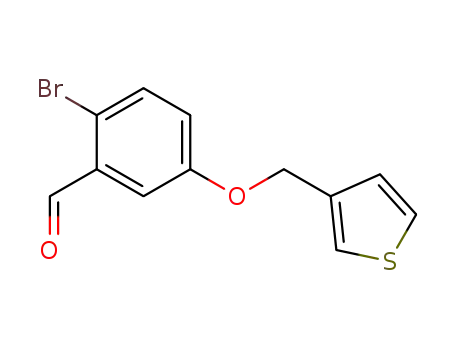
2-bromo-5-(3-thiophen-3-yl-methyloxy)benzaldehyde