Home > Products > intermediate
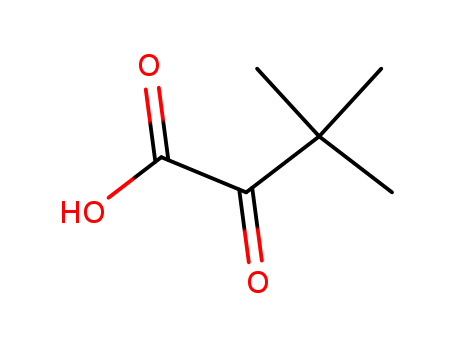







CasNo: 815-17-8
MF: C6H10O3
InChI:InChI=1/C6H10O3/c1-6(2,3)4(7)5(8)9/h1-3H3,(H,8,9)
N-substituted pantothenamides are analog...
The invention relates to the field of pe...
The invention discloses a novel synthesi...
The invention relates to the technical f...
An efficient Pd(OAc)2-catalyzed asymmetr...
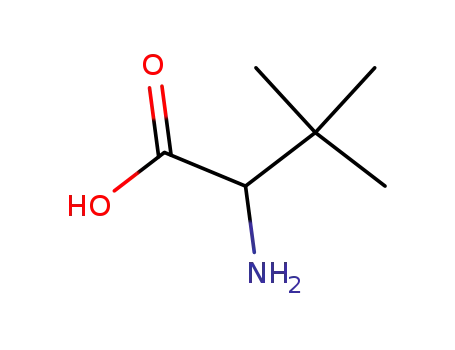
2-amino-3,3-dimethylbutanoic acid

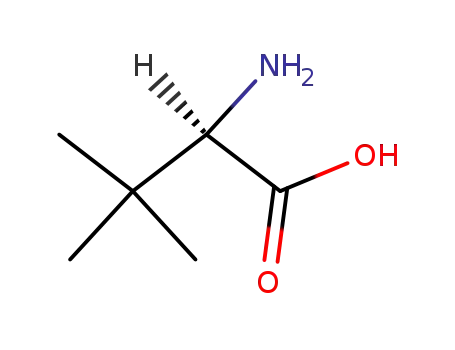
D-tert-leucine

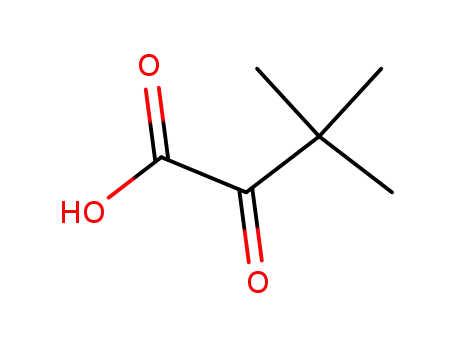
trimethylpyruvic acid
| Conditions | Yield |
|---|---|
|
With
NADH oxidase; leucine dehydrogenase; NAD;
at 30 ℃;
for 5h;
pH=8.0;
Enzymatic reaction;
|
![3,6-Di-tert-butyl-[1,2,4]trioxan-5-one](/upload/2025/4/926f504c-3486-4f53-9ebb-4ad68f2c4801.png)
3,6-Di-tert-butyl-[1,2,4]trioxan-5-one

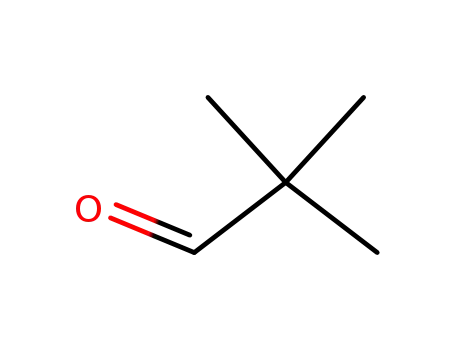
pivalaldehyde


trimethylpyruvic acid
| Conditions | Yield |
|---|---|
|
triethylamine;
In
dichloromethane;
at 20 - 25 ℃;
for 16h;
|
67% |
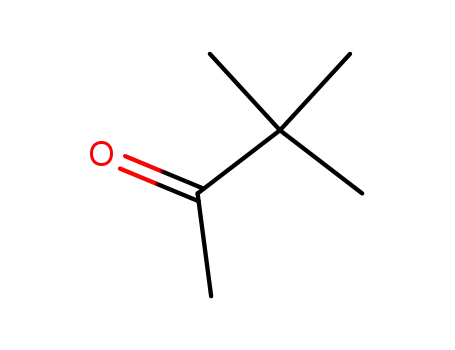
3,3-dimethyl-butan-2-one
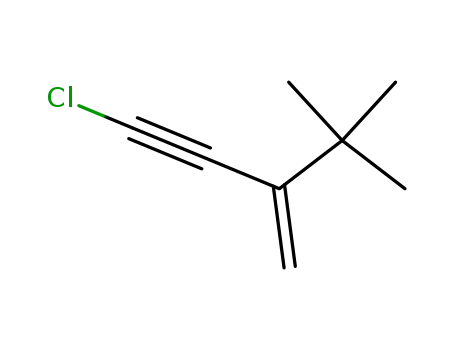
2-tert-butyl-4-chloro-but-1-en-3-yne
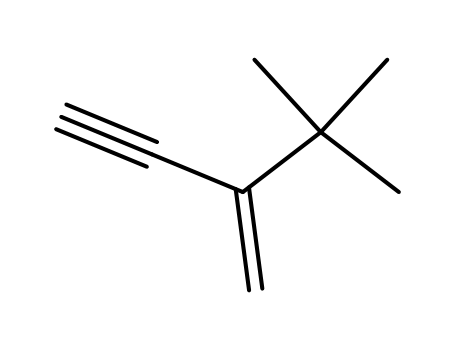
2-(tert-butyl)-but-1-en-3-yne
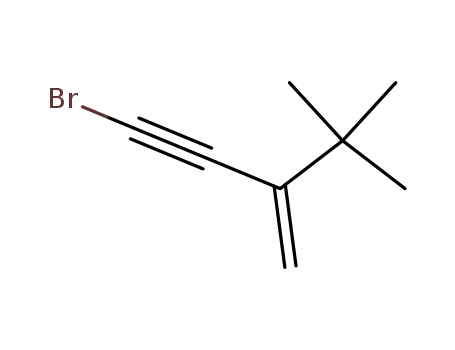
4-bromo-2-tert-butyl-but-1-en-3-yne
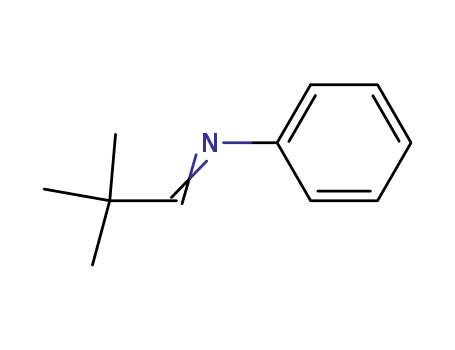
N-(neopentylidene)aniline
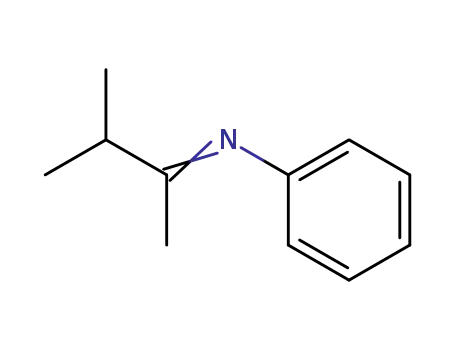
N-phenyl-(1,2-dimethylpropylidene)amine

pivalaldehyde
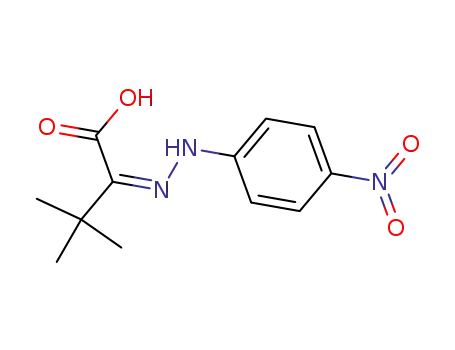
3,3-dimethyl-2-(4-nitro-phenylhydrazono)-butyric acid