Home > Products > intermediate







CasNo: 65277-42-1
MF: C26H28Cl2H4O4
Appearance: White powder
|
Antifungal drug |
Ketoconazole is a broad-spectrum antifungal imidazole with commercially available product being under the trade name of Jindakening, Meikangling and keNing. It interferes with the activity of fungal cytochrome P-450 with a high selectivity, thus inhibiting the biosynthesis of ergosterol in fungal cell membrane. It is effective in treating both shallow, deep fungal infections and can inhibit both fungal growth and the transition from spores to mycelium to prevent the further infection. It has antifungal effect on Candida genus, Fonsecaea, Coccidioides, Histoplasma, Sporothrix spp and Trichophyton. Clinically, it is suitable for the treatment of ringworm, athlete's foot, and skin ringworm, tinea, jock itch, and thrush, tinea versicolor as well as cutaneous candidiasis. Ketoconazole lotion, as a skin external use, is mainly used for clinical treatment and prevention of various kinds of infections caused by Malassezia such as tinea versicolor, seborrheic dermatitis and scalp pityriasis (dandruff), and can quickly alleviate the desquamation and itching caused by seborrheic dermatitis and scalp pityriasis. |
|
Pharmacological effects |
1. Pharmacology: ketoconazole belongs to azole-class antifungal drugs and has antifungal activity against various kinds of genus of deep fungal infections such as Candida, Fonsecaea, Coccidioides, Histoplasma, Sporothrix spp as well as Trichophyton. However, this product has a relative weak activity against Aspergillus, Sporothrix schenckii as well as some species of Dermateaceae and Mucor. This product, through actively interfering with the activity of cytochrome P-450, is capable of inhibiting the biosynthesis of the major steroids-ergosterol of the fungal cell membrane. Therefore, it destroys the fungal cell membrane and changes its permeability, resulting in the leakage of important intracellular material. Ketoconazole can also inhibit the biosynthesis of fungal triglyceride and phospholipid biosynthesis, inhibit the activity of oxidase and peroxidase, causing accumulation of intracellular hydrogen peroxide which further leads to cell submicroscopic structural degeneration and necrosis. For candida albicans, it can also suppress the transition process from spores to aggressive mycelium. 2. Toxicology: Long-term animal toxicity experiments have showed that ketoconazole can significantly increase the level of alkaline phosphatase and cause liver cell degeneration. |
|
Pharmacokinetics |
This product can be dissolved and absorbed in gastric acid. Upon the reduction of the acidity of gastric acid, its absorption can be reduced. Administration after meals can increase its absorption with the bioavailability of administration after meal being as high as 75%. After the single-dose oral administration of 200mg and 400mg, the peak plasma concentrations were 3.6 ± 1.65mg/L and 6.5 ± 1.44mg/L, respectively with the time for reaching peak being 1-4 hours. After the absorption, this product is widely distributed in the body and can reach the synovial fluid of inflammation, saliva, bile, urine, tendons, skin and soft tissue, feces and so on. It has a poor penetrating capability through the blood-brain barrier. In most cases, the drug concentration in the cerebrospinal fluid is less than 1mg/L. This product can also penetrate through the blood placental barrier. The binding rate of serum protein is about 90% or more with the elimination half-life being 6.5 to 9 hours. Some part of the drugs is subjected to metabolism in the liver through degradation into inactive imidazole ring and piperazine ring. The metabolites and prototype drug is mainly excreted through the bile. The drug excreted through the kidneys only accounts 13% of the administered dose, of which about 2% to 4% for drug prototype. The product can also be secreted into milk. |
|
Indications |
Ketoconazole is suitable for treating the following systemic fungal infections: 1. Candidiasis, chronic mucocutaneous candidiasis, oral candidiasis infection, Candida urinary tract infections as well as chronic, recurrent vaginal candidiasis which can be cured by topical therapy. 2. Dermatitis blastomycosis. 3. Coccidioidomycosis. 4. Histoplasmosis. 5. Chromomycosis. 6. Paracoccidioidomycosis. It can be used for treating fungal skin diseases, hair ringworm and tinea versicolor caused by fungi and yeasts. When local therapy or oral administration of griseofulvin is invalid, or griseofulvin is unacceptable in the treatment of severe refractory fungal skin infection, we can choose the treatment of this drug. The above information is edited by the lookchem of Dai Xiongfeng. |
|
Side effects |
External administration 1. Common erythema, burning, itching, stinging or other irritation, folliculitis, skin atrophy and thinning as well as telangiectasia. 2. It can be observed of dry skin, hirsutism, striae atrophicae and increased susceptibility to infection. 3. Long-term medication may cause cortex hyperthyroidism, manifested as hirsutism, acne, moon face, osteoporosis and other symptoms. 4. It can be occasionally observed of allergic contact dermatitis. Side effects of oral administration 1. Hepatotoxicity: ketoconazole can cause increased serum aminotransferase (AST, ALT) level and is reversible. It can be occasionally observed of severe liver toxicity, primarily being liver cell type with the incidence being about 0.01%. The clinical manifestations include jaundice, dark urine, white-color faeces and abnormal fatigue, etc., these effects can usually resume after the withdrawal of the drug, but there are also cases of deaths; there are also cases of hepatitis in children. 2. Gastrointestinal reaction: nausea, vomiting and anorexia are common cases. 3. Gynecomastia and lack of semen; this is related to the effect of this product on suppression of the biosynthesis of testosterone and adrenal cortical hormone. |
|
Precautions |
1. Take it with caution upon the following cases: lack of gastric acid (may cause the reduction of the absorption of the product). Alcoholism or liver damage (it can cause liver toxicity). 2.Before or during the treatment, the patients should be regularly subject to monitoring of liver function. Elevated serum aminotransferase may not be accompanied by symptoms of hepatitis, however, if the serum aminotransferase value continues to rise or increase, or associated with liver toxicity symptoms, we should discontinue ketoconazole treatment. 3. For simultaneous administration of cimetidine or furan thiamine, take them at least 2 hours after taking this drug. 4. This product can cause photosensitivity reactions. Therefore, during the medication, we should avoid prolonged exposure to bright light and can wear colored glasses. 5. It is not allowed to take alcoholic beverages while taking the drug. Pay attention if dizziness or drowsiness occurs. 5. Patients of renal dysfunction don’t need to be subject to reduced dose upon taking it. 6. Ketoconazole has a very poor capability of penetrating blood-brain barrier and is not suitable for the treatment of fungal meningitis. This product also has poor efficacy in treating Aspergillus, Mucor or maduromycosis and thus is also not suitable. 7. Interfere with the diagnosis: can cause elevated serum aminotransferase, can also cause increased level of hemobilirubin. |
|
Pregnant and lactating women |
The product can penetrate through the blood placental barrier. Animal experiments have shown that the product can be teratogenic such as syndactylia, lack of finger (toe) and dystocia in rats. US FDA data has shown that the application of this drug in pregnant women should be classified into Class C, namely being toxic in animal studies but is lack of adequate information in human studies. Therefore, pregnant women should be avoided for using it. Ketoconazole can be secreted into breast milk. The administration of it for humans has not found any issues, but the product can increase the likelihood of the occurrence of neonatal kernicterus, lactating women should weigh both advantages and disadvantages for using it. |
|
Production method |
Put the mixture containing 2.4 parts of 1-acetyl-4-(4-hydroxyphenyl) piperazine, 0.4 part of 78% sodium hydride, 75 parts of dimethyl sulfate, 22.5 parts of benzene at 40 ℃ for stirring of 1 hour, followed by addition of 4.2 parts of 2-(2,4-dichlorobenzyl-2-(1H-imidazol-1-yl-methyl)-1,3-dioxolane-4-ylmethyl methanesulfonate. Stir at 100 °C overnight with the reaction product resulting in 3.2 parts ketoconazole after treatment. |
|
Therapeutic Function |
Antifungal |
|
World Health Organization (WHO) |
Ketoconazole, an imidazole antifungal agent, was introduced in 1978 for the topical and systemic treatment of a wide variety of fungal infections. Its use by mouth has been associated with hepatotoxicity, including cases of hepatitis, which have usually been reversible on discontinuation of the drug, but some fatalities have also occurred. Ketoconazole is widely marketed. |
|
Antimicrobial activity |
The spectrum includes dermatophytes, some dimorphic fungi and Candida spp. |
|
Acquired resistance |
Resistance has been documented in patients treated for chronic mucocutaneous candidosis and AIDS patients with oropharyngeal or esophageal candidosis. Some fluconazoleresistant C. albicans and C. glabrata are cross-resistant to ketoconazole. |
|
Pharmaceutical Applications |
A synthetic dioxolane imidazole available for oral and topical use. |
|
Biological Activity |
Antifungal agent; potent inhibitor of cytochrome P450c17. |
|
Biochem/physiol Actions |
Ketoconazole is an imidazole derivative. It plays an important role in inhibiting the conversion of lanosterol to ergosterol in the cell wall of fungi. Ketoconazole has therapeutic effects against dermatophytosis, superficial candidiasis, and paracoccidioidomycosis. |
|
Mechanism of action |
Ketoconazole has little effect on Aspergillus or Cryptococcus. Ketoconazole is highly dependent on low stomach pH for absorption, and antacids or drugs that raise stomach pH will lower the bioavailability of ketoconazole. As with other azoles, it is extensively metabolized by microsomal enzymes. All the metabolites are inactive. Evidence that CYP3A4 plays a significant role in metabolism of ketoconazole is that coadministration of CYP3A4 inducers, such as phenytoin, carbamazepine, and rifampin, can cause as much as a 50% reduction in levels of ketoconazole. |
|
Synthesis |
Ketoconazole, cis-1-acetyl-4-[4-[2-(2,4-dichlorophenyl)-2-(1H-imidazole- 1-ylmethyl)-1,3-dioxolan-4-ylmethyl]phenyl]piperazine (35.2.4), is synthesized from 2,4-dichlorophenacyl bromide, the ketalization of which using glycerol gives cis-2-(2,4- dichlorophenyl)-2-bromoethyl-4-hydroxymethyl-1,3-dioxolane (35.2.1). Acylating the hydroxyl group of this compound with benzoyl chloride, and then alkylating the resulting compound with imidazole gives the derivative (35.2.2). Next, alkaline hydrolysis removes the benzoyl group, and a reaction with methanesulfonyl chloride gives a mesylate (35.2.3). Finally, alkylating the resulting 1-acetyl-4-(4-hydroxyphenyl)piperazine gives ketoconazole (35.2.4). |
|
Veterinary Drugs and Treatments |
Because of its comparative lack of toxicity when compared to amphotericin B, oral administration, and relatively good efficacy, ketoconazole has been used to treat several fungal infections in dogs, cats, and other small species. Ketoconazole is often employed with amphotericin B to enhance the efficacy of ketoconazole, and by reducing the dose of amphotericin B, decreasing its risk of toxicity. See the Dosage section or Pharmacology section for specifics. Newer antifungal agents (fluconazole, itraconazole) have advantages over ketoconazole, primarily less toxicity and/or enhanced efficacy; however, ketoconazole can be significantly less expensive than the newer agents. Ketoconazole is considered by some to still be the drug of choice for treating histoplasmosis in dogs. Use of ketoconazole in cats is controversial and some say it should never be used that species. Ketoconazole is also used clinically for the medical treatment of hyperadrenocorticism in dogs. Ketoconazole appears to be a viable option (although relatively expensive) to mitotane, particularly for palliative therapy in dogs with large, malignant, or invasive tumors where surgery is not an option. Ketoconazole is also used frequently in dogs for stabilization prior to surgery. It is a reversible inhibitor of steroidogenesis, so it is usually not a viable option for long-term treatment. Because it interferes with the metabolism of cyclosporine, it has been used to reduce the dosage necessary for cyclosporine in dogs. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Aminophylline and theophylline; possibly increased concentration of aminophylline and theophylline. Analgesics: inhibits buprenorphine metabolism - reduce buprenorphine dose; possible increased risk of ventricular arrhythmias with methadone - avoid; increases oxycodone and sufentanil concentration; avoid with paracetamol. Anti-arrhythmics: increased risk of ventricular arrhythmias with disopyramide - avoid; concentration of dronedarone increased - avoid. Antibacterials: metabolism increased by rifampicin; may reduce rifampicin concentration; concentration of bedaquiline increased - avoid; avoid with fidaxomicin; concentration possibly reduced by isoniazid; avoid with clarithromycin and telithromycin in severe renal (both) and hepatic impairment (telithromycin only). Anticoagulants: anticoagulant effect of coumarins enhanced; concentration of apixaban, dabigatran and rivaroxaban increased - avoid; concentration of edoxaban increased - reduce edoxaban dose. Antidepressants: avoid concomitant use with reboxetine; ketoconazole increases concentration of mirtazapine. Antidiabetics: concentration of pioglitazone, saxagliptin and tolbutamide increased. Antiepileptics: concentration of ketoconazole reduced by fosphenytoin and phenytoin and possibly carbamazepine; concentration of perampanel and possibly carbamazepine increased. Antifungals: concentration of isavuconazole increased - avoid. Antihistamines: concentration of loratidine possibly increased - avoid; avoid with mizolastine; concentration of rupatadine increased. Antimalarials: avoid with piperaquine with artenimol and artemether and lumefantrine; concentration of mefloquine increased. Antimuscarinics: absorption of ketoconazole reduced; concentration of darifenacin increased - avoid; reduce dose of fesoterodine; concentration of oxybutynin and solifenacin increased; avoid with tolterodine. Antipsychotics: increased risk of ventricular arrhythmias with pimozide - avoid; possibly increased concentration of quetiapine - reduce quetiapine dose; inhibits aripiprazole metabolism - reduce aripiprazole dose; concentration of lurasidone increased - avoid |
|
Metabolism |
Ketoconazole is extensively degraded by the liver, and very little active drug is excreted in either the urine or bile; the dose need not be modified for renal insufficiency. Adverse reactions to topical ketoconazole are very rare. |
|
Pharmacological Activity |
(+)-Ketoconazole is an imidazole antifungal agent with some antibacterial activity. It may act by interfering with the permeability of the fungal cell membrane. |
|
Dosage Forms |
Formulated into various dosage forms for oral and topical administration. |
|
Chemical Stability |
Ketoconazole is a weak base with two pKa values. |
|
Inhibition of Cytochrome P450-3A (CYP3A) |
Widely used as an inhibitor of human cytochrome P450-3A isoforms. |
|
Clinical Use and Adverse Effects |
First broad-spectrum oral antifungal agent for systemic and superficial mycoses. |
|
Monitoring and Management |
The use of ketoconazole should be closely monitored, especially with liver enzyme monitoring, due to potential hepatotoxicity. Hepatotoxicity associated with ketoconazole use is usually mild and resolves after discontinuation of the drug. |
|
Brand name |
Ketozole (Taro); Nizoral (Janssen); Nizoral (McNeil);Cerozalol;Cetonax;Fetonal;Fungarol;Fungo-hubber;Ketocidin;Ketoisdin;Ketonan;Ketoral;Micoral;Micotek;Micoticum;Nizcrem;Nizoral 2% shampoo;Nizoral 20% cream;Nizovules;Nizshampoo;Oromycosal;Oronazol;Panfungol;Rofenid;Spike;Unidox. |
|
General Description |
Ketoconazole is an imidazole antifungal agent administered through topical or oral means. It is used for the treatment of chronic mucocutaneous candidiasis, fungal infections of the gastro-intestinal tract, dermatophyte infections, systemic infections, and fungal infections in immuno-compromised patients. |
InChI:InChI=1/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23?,26-/m0/s1
The invention belongs to the technical f...
The graphene oxide (GO) was covalently c...
The present study describes a generic st...
This work aimed to develop a chiral sepa...
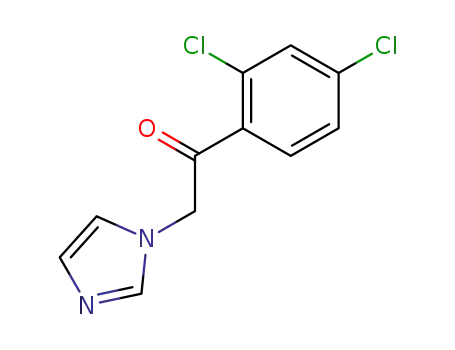
1-(2,4-dichlorophenyl)-2-(imidazol-1-yl)ethanone

(+/-)-1-acetyl-4-<4-(2,3-dihydroxypropoxy)phenyl>piperazine

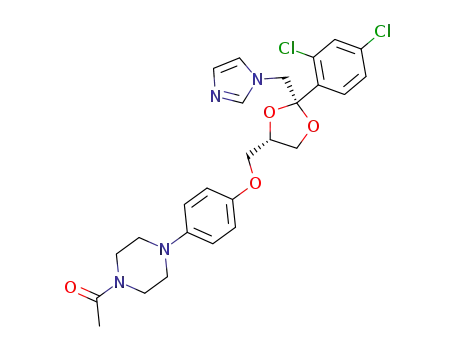
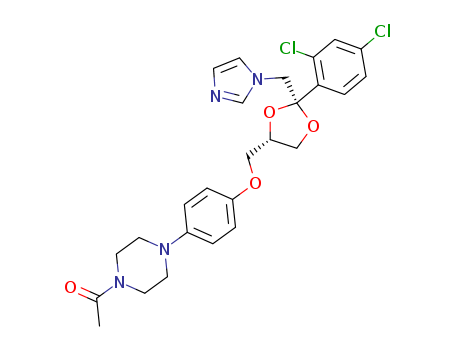
ketoconazole
| Conditions | Yield |
|---|---|
|
With
trifluoroacetic acid;
In
dimethyl sulfoxide; toluene;
for 16h;
Reagent/catalyst;
Temperature;
Reflux;
|
80% |
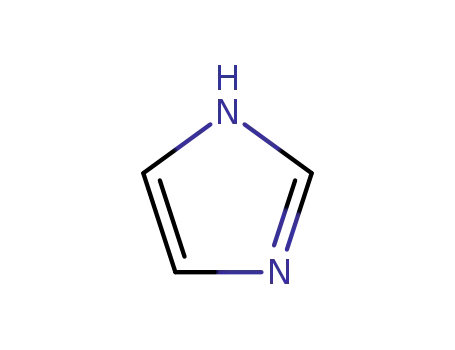
1H-imidazole

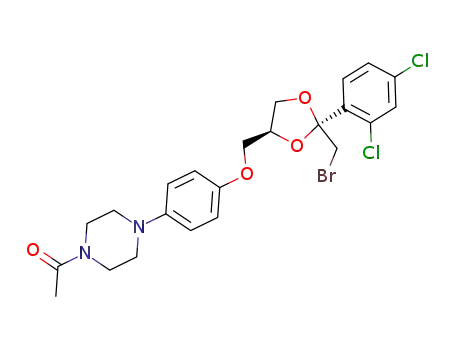
(2R,4S)-cis-2-(bromomethyl)-2-(2,4-dichlorophenyl)-4-<<4-(4-acetylpiperazin-1-yl)phenoxy>methyl>-1,3-dioxolane


ketoconazole

(2R,4S)-cis-2-(hydroxymethyl)-2-(2,4-dichlorophenyl)-4-<<4-(4-acetylpiperazin-1-yl)phenoxy>methyl>-1,3-dioxolane
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
N,N-dimethyl acetamide;
for 4h;
Heating;
|
10% 53% |

1H-imidazole

(2R,4S)-cis-2-(bromomethyl)-2-(2,4-dichlorophenyl)-4-<<4-(4-acetylpiperazin-1-yl)phenoxy>methyl>-1,3-dioxolane
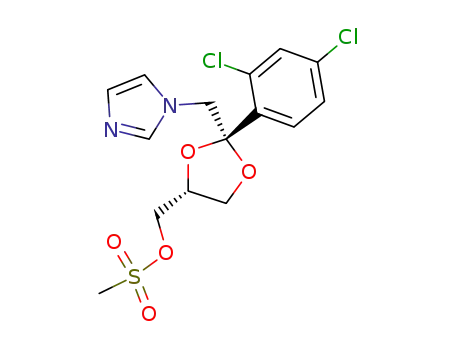
(2R,4R)-(+)-<2-(2,4-dichlorophenyl)-2-<(1H-imidazol-1-yl)methyl>-1,3-dioxolan-4-yl>methyl methanesulfonate
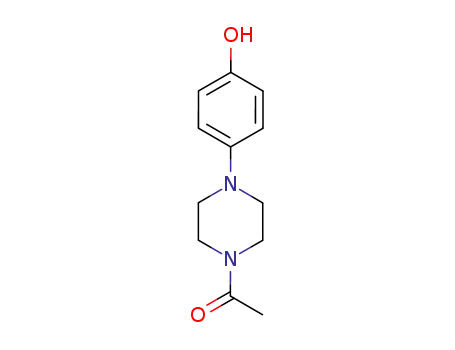
N-acetyl-N'-(4-hydroxyphenyl)piperazine
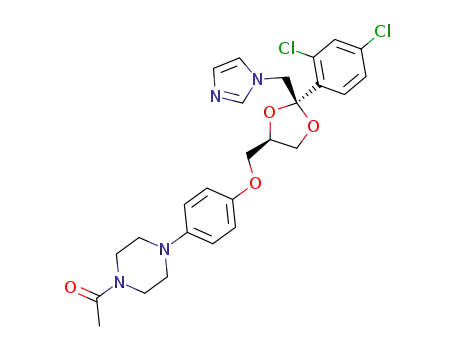
ketoconazole
2,4-diamino-6-piperidinopyrimidine 3-oxide