Home > Products > intermediate
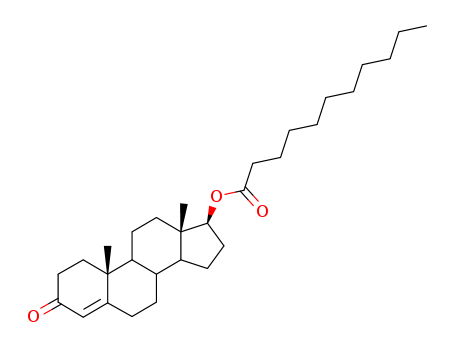







CasNo: 5949-44-0
MF: C30H48O3
Appearance: White crystalline powder
|
Indications |
JATENZO (testosterone undecanoate) is an androgen indicated for testosterone replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone: Primary hypogonadism (congenital or acquired): testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, orchiectomy, Klinefelter syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone concentrations and gonadotropins (follicle-stimulating hormone [FSH], luteinizing hormone [LH]) above the normal range. Hypogonadotropic hypogonadism (congenital or acquired): gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency or pituitary-hypothalamic injury from tumors, trauma, or radiation. These men have low testosterone serum concentrations but have gonadotropins in the normal or low range.www.accessdata.fda.gov |
|
Pharmacokinetics |
Testosterone undecanoate is a prodrug of testos-terone esterified in the 17b-position with undecanoic acid. Following oral administration, thisprodrug is metabolized only partly in the intestinal wall, and the remaining fraction of testosterone undecanoate is absorbed via the lymphatic system, and hence, avoids first pass hepatic metabolism. After reaching the systemic circulation, testosterone undecanoate rapidly converts to free testosterone. The absolute bioavailability of testosterone following oral administration of testosterone undecanoate is estimated to be 7%. Recent studies in lymph duct cannulated dogs revealed that all of the testosterone undecanoate absorbed into the systemic circulation is due to lymphatic absorption of the prodrug, and more than 80% of the free testosterone in the plasma is contributed by systemic hydrolysis of the lymphatically transported prodrug. |
|
Side effects |
Side effects of testosterone undecanoate include symptoms of masculinization like acne, increased hair growth, voice changes, hypertension, elevated liver enzymes, hypertriglyceridemia, and increased sexual desire. The drug is a prodrug of testosterone, the biological ligand of the androgen receptor (AR) and hence is an androgen and anabolic steroid. It has strong androgenic effects and moderate anabolic effects, which make it useful for producing masculinization and suitable for androgen replacement therapy. Testosterone undecanoate is a testosterone ester and a prodrug of testosterone in the body. Because of this, it is considered to be a natural and bioidentical form of testosterone. |
|
Definition |
ChEBI: Testosterone undecanoate is a steroid ester. It is a metabolite of Testosterone. It is a promising androgen for male hormonal contraception. |
InChI:InChI=1/C30H48O3/c1-4-5-6-7-8-9-10-11-12-28(32)33-27-16-15-25-24-14-13-22-21-23(31)17-19-29(22,2)26(24)18-20-30(25,27)3/h21,24-27H,4-20H2,1-3H3
The invention discloses a preparation me...
The invention discloses a synthesis meth...
The invention provides a method for synt...
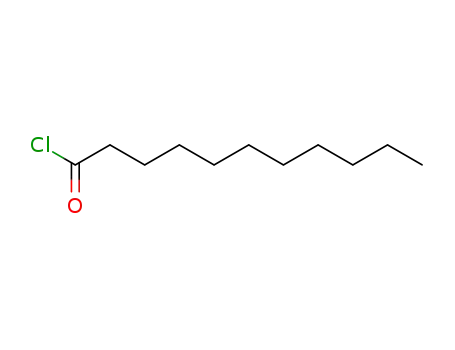
undecanoyl chloride

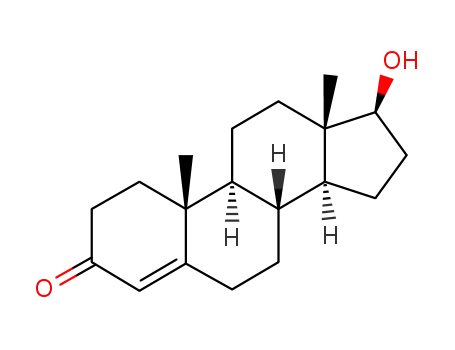
testosterone

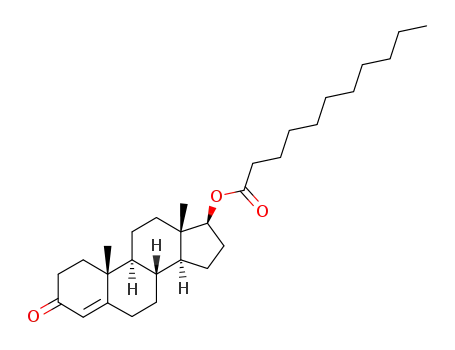
testosterone undecanoate
| Conditions | Yield |
|---|---|
|
With
pyridine;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
for 1h;
Solvent;
Reagent/catalyst;
Inert atmosphere;
|
96% |
|
With
dmap; N-ethyl-N,N-diisopropylamine;
In
1,2-dichloro-ethane;
at 40 ℃;
|
13.5 g |

undecanoyl chloride

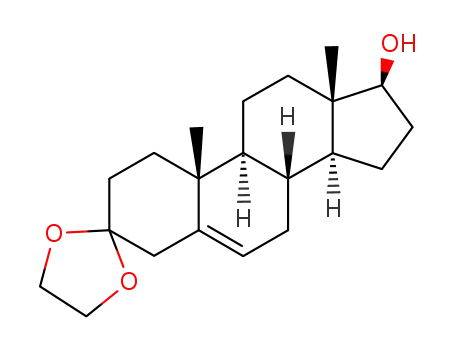
3,3-(ethylenedioxy)-5-androsten-17β-ol


testosterone undecanoate
| Conditions | Yield |
|---|---|
|
undecanoyl chloride; 3,3-(ethylenedioxy)-5-androsten-17β-ol;
With
dmap; triethylamine;
In
dichloromethane;
at 20 ℃;
Inert atmosphere;
With
hydrogenchloride;
In
water;
|
96.15% |

undecanoyl chloride

testosterone
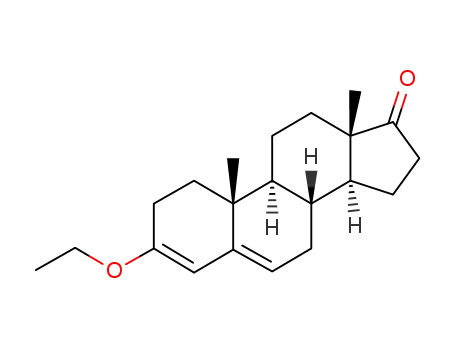
3-ethoxyandrosta-3,5-dien-17-one
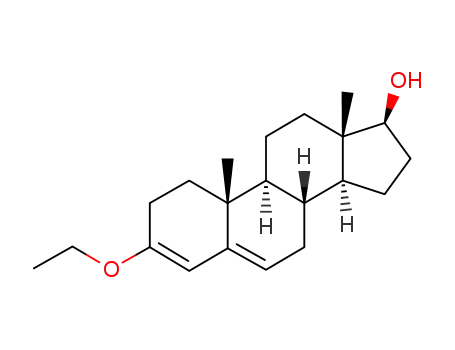
17β-hydroxy-3-ethoxyandrosta-3,5-diene