Home > Products > intermediate
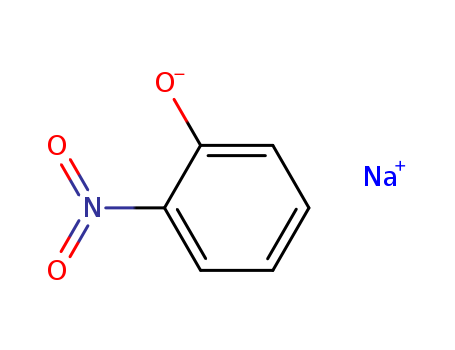







CasNo: 824-39-5
MF: C6H4NNaO3
Appearance: dark red acicular crystals
|
General Description |
Sodium 2-nitrophenoxide, also known as sodium o-nitrophenolate, is an organic compound primarily used as a plant growth regulator. Chemically, it's a sodium salt of 2-nitrophenol, created through the process of nitration of phenol. Its structural formula is C6H4NO3Na. Sodium 2-nitrophenoxide helps stimulate the growth of crops by enhancing the rate of photosynthesis, promoting the absorption of nutrients and improving resistance to pests and diseases. It's generally used in the agriculture industry for crops like wheat, cotton, and fruit trees. Moreover, due to its nitro group, it can also act as a strong reducing agent. Overexposure can potentially cause harm to the environment or human health. |
InChI:InChI=1/C6H5NO3.Na/c8-6-4-2-1-3-5(6)7(9)10;/h1-4,8H;/q;+1/p-1
A supramolecular catalyst of Pd/β-cyclod...
The nucleophilicity of both ortho- and m...
The invention belongs to the technical f...
About 30 analogues of BAPTA-AM, a potent...
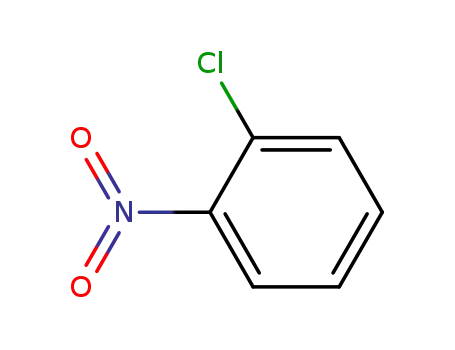
2-Chloronitrobenzene

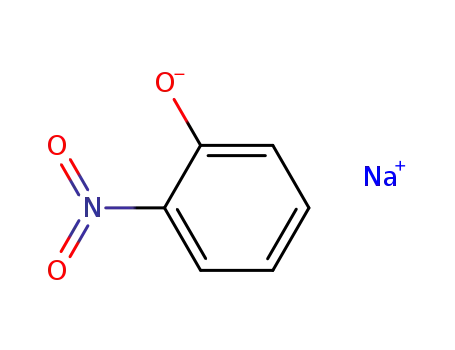
sodium o-nitrophenate
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
at 160 - 175 ℃;
for 4.5h;
under 51005.1 - 54005.4 Torr;
Large scale;
|
1.2 t |
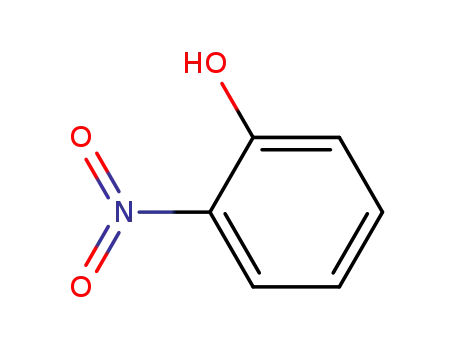
2-hydroxynitrobenzene


sodium o-nitrophenate
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
ethanol;
|
|
|
With
sodium hydroxide;
In
water;
at 20 ℃;
for 1h;
|
|
|
With
sodium tetrahydroborate;
In
water;
|
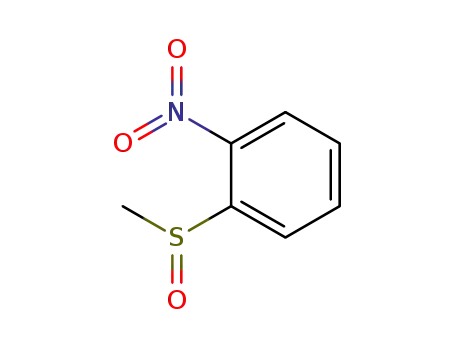
methyl-(2-nitro-phenyl)-sulfoxide
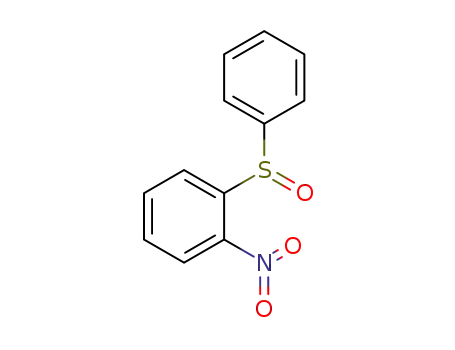
1-nitro-2-(phenylsulfinyl)benzene
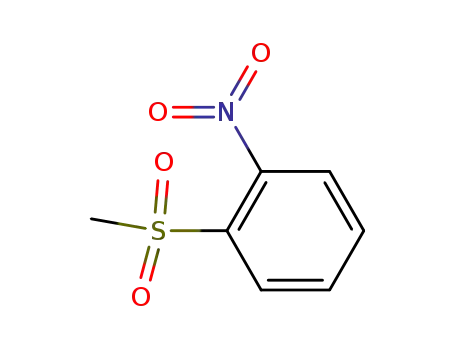
1-(methylsulfonyl)-2-nitrobenzene
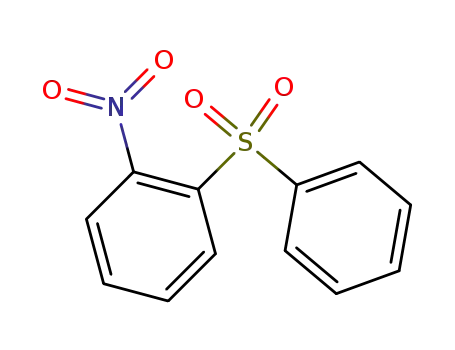
1-nitro-2-(phenylsulfonyl)benzene
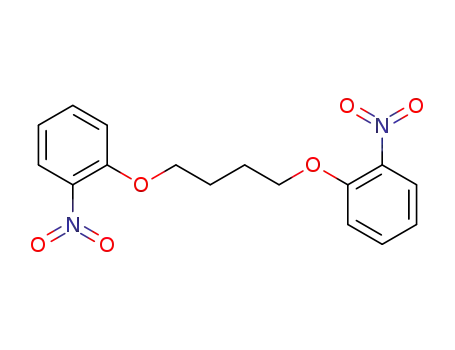
1,6-bis(2-nitrophenyl)-1,6-dioxahexane
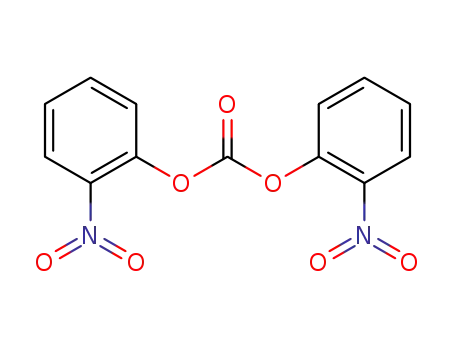
mononitrophenyl carbonate
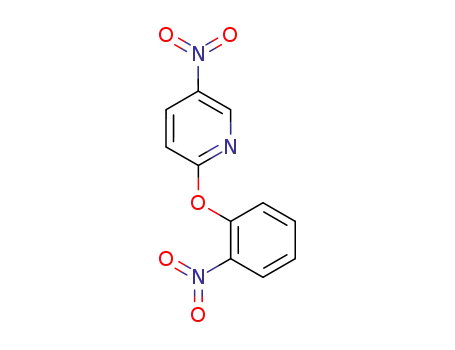
5-nitro-2-(2-nitro-phenoxy)-pyridine
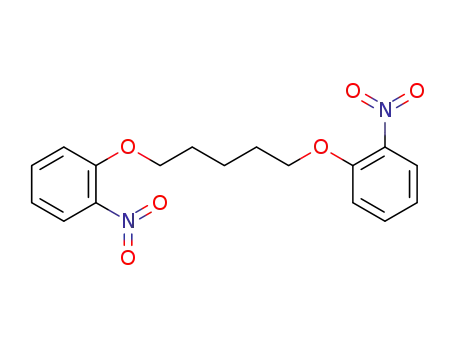
1,7-bis(2-nitrophenyl)-1,7-dioxaheptane