Home > Products > intermediate
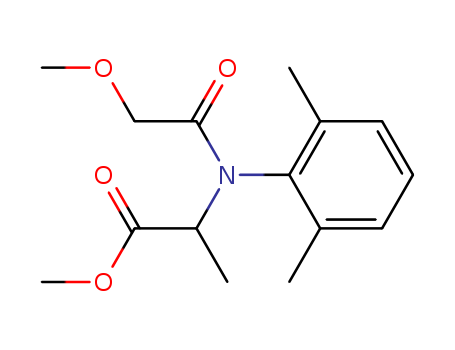







CasNo: 57837-19-1
MF: C15H21NO4
Appearance: Colorless, odorless crystal
|
Hazard |
Moderately toxic by ingestion. |
|
Trade name |
AGROX? PREMIERE; ALLEGIENCE?; APRON?; CG 117?; CGA-48988?; CHLORAXYL?; COTGUARD?; EPERON?; DELTA-COAT; FOLIO? GOLD; GAUCHO?; KODIAK?; METALAXIL?; METAXANIN?; PACE?; PREVAIL?; RAXIL? (tebu- conazole + metalaxyl); RIDOMIL? GOLD/BRAVO?; RIDOMIL?; RIDOMIL 2E?; SUBDUE? |
|
Pharmacology |
In mycelium of Phytophthora megasperma,metalaxyl affected primarily rRNA synthesis (polymerase I), whereas mRNA was much less sensitive; therefore, inhibition of rRNA synthesis is considered as the primary site of action of PAFs (23). The PAFs exhibit strong preventive and curative activity. They affect especially hyphal growth (inside and outside the plant tissue) as well as haustorium and spore formation (15). Although not fully utilized for resistance management reasons, PAFs also exhibit strong eradicative and antisporulant activity in the disease cycle of target pathogens. On the other hand, PAFs do not inhibit the early stages in the disease cycle like zoospore release, spore germination, and penetration of the host tissue (15). Because spores contain many ribosomes to support early growth stages, RNA synthesis is fully operating only after spore germination; later development stages are therefore most sensitive to PAFs (23). As a consequence of RNA inhibition, the precursors of RNA synthesis (i.e., nucleoside triphosphates) are accumulated; they activate β-1,3-glucansynthetases, which are involved in cell wall formation (23). Metalaxyl-treated hyphae often produce thicker cell walls than do untreated ones. |
|
Safety Profile |
Moderately toxic by ingestion. When heated to decomposition it emits toxic fumes of NOx. |
|
Potential Exposure |
Metalaxyl is phenylamide systemic fungicide used on a variety of food and nonfood crops including tobacco, turf and conifers, and ornamentals. Used in combination with fungicides of different mode of action as a foliar spray on tropical and subtropical crops; as a seed treatment to control downy mildew; and as a soil fumigant to control soil-borne pathogens. Banned for use in EU. |
|
Environmental Fate |
Soil. Little information is available on the degradation of metalaxyl in soil; however, Sharom and Edgington (1986) reported metalaxyl acid as a possible metabolite. Repeated applications of metalaxyl decreases its persistence. Following an initial application, the average half-life was 28 days. After repeated applications, the half-life decreased to 14 days (Bailey and Coffey, 1985).Carsel et al. (1986) studied the persistence of metalaxyl in various soil types. The application rate was 2.2 kg/ha. In a fine sand, metalaxyl concentrations at soil depths of 15, 20, 45 and 60 cm were 100, 150, 100 and 75 ppb, respectively, 55 days afterPlant. In plants, metalaxyl undergoes ring oxidation, methyl ester hydrolysis, ether cleavage, ring methyl hydroxylation and N-dealkylation (Owen and Donzel, 1986). Metalaxyl acid was identified as a hydrolysis product in both sunflower leaves anIn pigeon peas, metalaxyl may persist up to 12 days (Indira et al., 1981; Chaube et al., 1984). |
|
Metabolic pathway |
O-Demethylation is one of the major routes of metalaxyl degradation in the plant cell suspension culture. Although hydroxylation of methyl groups in the phenyl ring predominates in both lettuce and grapes, species differences are evident in grapes, whereas N-dealkylation and aryl hydroxylation are less important in lettuce. Two isomeric metabolites of methyl hydroxylation and the hydroxylated metabolite of the phenyl ring are identified as fungus metabolites. By UV irradiation of metalaxyl in aqueous solution, two rearrangement products of the N-acyl group to the 4-position on the phenyl ring are identified. |
|
Metabolism |
The degradation pathways of pesticides are published in the "FAO Plant Production and Protection Papers." Because the degradation pathways are similar for all PAF). In plants, metalaxyl is metabolized by four types of phase I reaction to form eight metabolites; at phase II, most of the metabolites are sugar-conjugated. The types of reaction in phase I are hydroxylation at the phenyl ring, oxidation of one of the tolylic methyl groups (Formula d), hydrolysis of the methyl ester (Formula e), and ether cleavage (Formula b). In phases II and III, there is also a dealkylation of the nitrogen (Formula l), in addition to the combination of the above-mentioned reaction types forming the compounds of Formulas f, h, and m. In mammals, following oral administration, metalaxyl is rapidly absorbed and rapidly and almost completely eliminated with urine and feces. Metabolism proceeds via the same degradation pathways as in plants, leading to products containing an oxidized tolylic methyl group with or without the hydrolyzed ester function (Formulas d, h, and i, respectively) containing a dealkylated nitrogen and a hydroxy group formed by ether cleavage (Formula l via b or e/f), containing an oxalyl function formed by ether cleavage followed by oxidation of the generated alcohol (Formula c), and containing the hydroxylated phenyl ring (Formula a). Residues in tissues were generally low, and there was no evidence for accumulation or retention of metalaxyl or its metabolites. In soil, similar degradation products are found as in plants and animals with the exception of three additional products of Formulas k, n, and g. |
|
Shipping |
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required. |
|
Toxicity evaluation |
If used according to label recommendations, PAFs are considered to be safe to humans, animals, and the environment. The active ingredients represent only low-to-moderate acute oral and dermal hazard to rats, mice, and rabbits. The compounds do not exhibit mutagenic, oncogenic, and teratogenic hazards. No or only weak (furalaxyl, ofurace) skin irritant potential exists in rabbits and no skin sensitization is present in guinea pigs, whereas some compounds are weak to serious eye irritants in rabbits (except benalaxyl and oxadixyl). In long-term toxicity studies, the "no-observableeffect level" (NOEL) in rats is 2.5 mg/kg body weight/day for metalaxyl, metalaxyl-M, and ofurace; 5 mg/kg for benalaxyl; and 11 mg/kg for oxadixyl, whereas in dogs, the NOEL is 8.0 mg/kg body weight/day for metalaxyl and metalaxyl-M, 7 mg/kg for benalaxyl, and 12 mg/kg for oxadixyl. Using a safety factor of 100, the "acceptable daily intake" (ADI) for PAFs ranges from 0.025 to 0.11 mg/kg. The PAFs are unlikely to pose any toxicological risk to birds (bobwhite quail, mallard ducks), fish (rainbow trout, carp), honeybees, earthworms, Daphnia, and algae. The observed LD50 (LC, EC) values are very favorable for all PAFs; only benalaxyl shows lower figures in respect to earthworm, Daphnia, and algae. |
|
Degradation |
Metalaxyl is very stable in neutral and acidic media at room temperature and it is reasonably stable to aqueous photolysis. Its calculated half-lives in buffers at 25 °C below pH 7 are <3 years and at pH 9, 12 weeks. Only at pH 11 was measurable hydrolysis seen (half-life 1.6 days) (Melkebeke et al., 1986). Thus environmental degradation can be expected to be slow. [14C-phenyl]Metalaxy1ir radiated in aqueous solution with UV light at 30 °C was degraded with a half-life of 2-3 days at four pH values between 2.8 and 8.8. Acetone (1%) accelerated the rate of decomposition. Two rearrangement products (2 and 3) were isolated at pH 6.8; these accounted for 3 and 6% of the radioactivity, respectively. Irradiation of 2 showed that it was a precursor of compound 3 (Yao et al., 1989). Though these products appear to be unusual, there is a precedent for such reactions and the structures were determined by 1H and 13C NMR spectroscopy. Decomposition under simulated sunlight was slower with a half-life of 297 days (Pirisi et al., 1996). Amide bond cleavage and N-dealkylation to compounds 4 and 5 was reported. The dimethylaniline (6) was a putative product but a separate experiment showed that it was degraded at a higher rate than the parent and so was not observed from metalaxyl. The products are shown in Scheme 1. |
|
Incompatibilities |
Incompatible with alkaline materials, strong acids, oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic, and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). |
|
Waste Disposal |
Small amounts may be destroyed by alkaline hydrolysis. Admixture with alkali can be followed by soil burial. Larger quantities can be disposed of by incineration in admixture with acetone or xylene and using effluent gas scrubbing. Do not reuse empty container; proper disposal required. |
|
Agricultural Uses |
Fungicide: Metalaxyl is used as a systemic fungicide on a variety of food and non-food crops including tobacco, turf and conifers, and ornamentals. Used in combination with fungicides of different mode of action as a foliar spray on tropical and subtropical crops; as a seed treatment to control downy mildew; and as a soil fumigant to control soilborne pathogens. |
InChI:InChI=1/C15H21NO4/c1-10-7-6-8-11(2)14(10)16(13(17)9-19-4)12(3)15(18)20-5/h6-8,12H,9H2,1-5H3
The compound of Chemical Formula 1 and t...
The invention discloses a synthesis meth...
In this study, magnetic multiwalled carb...
Core-shell silica microspheres with a na...
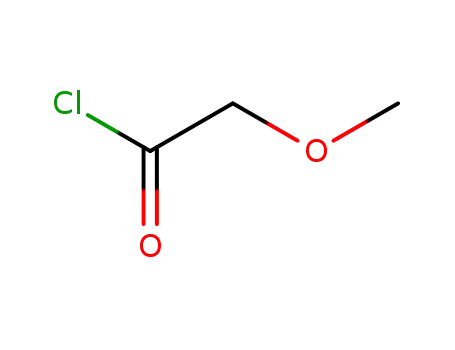
Methoxyacetyl chloride

![methyl (2R)-2-[(2,6-dimethylphenyl)amino]propanoate](/upload/2025/4/908ac010-bc85-45fd-bb67-a819ce765335.png)
methyl (2R)-2-[(2,6-dimethylphenyl)amino]propanoate

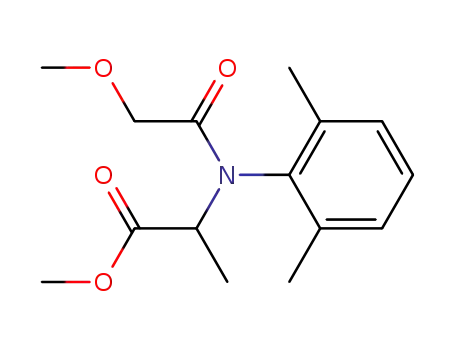
methyl-N-(methoxyacetyl)-N-(2,6-xylyl)-DL-alaninate
| Conditions | Yield |
|---|---|
|
In
toluene;
for 2.5h;
Reflux;
|
88.2% |
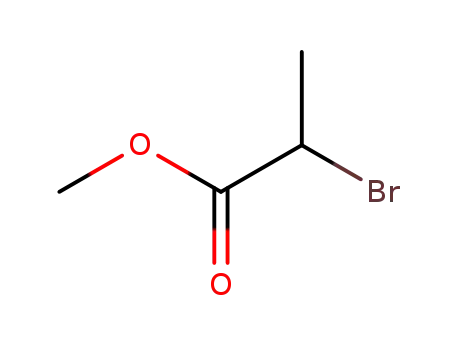
Methyl 2-bromopropionate

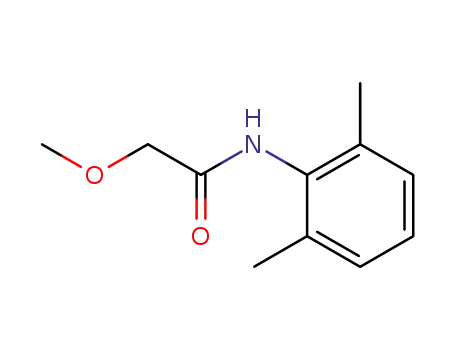
N-(1-methoxyacetyl)-2,6-dimethylaniline

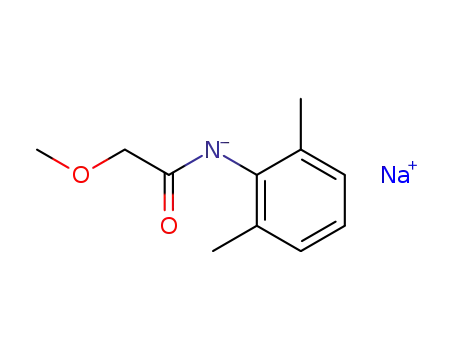
N-methoxyacetyl-2,6-dimethylaniline sodium salt


methyl-N-(methoxyacetyl)-N-(2,6-xylyl)-DL-alaninate
| Conditions | Yield |
|---|---|
|
With
sodium methylate;
In
5,5-dimethyl-1,3-cyclohexadiene;
|
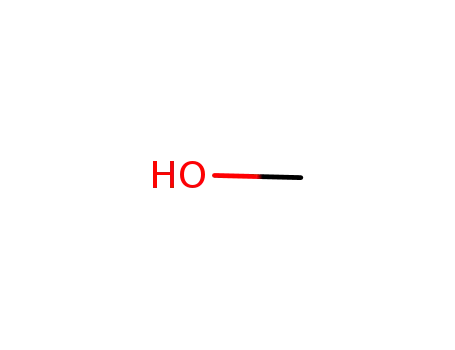
methanol
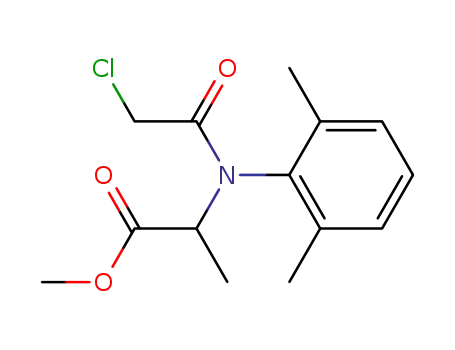
methyl 2-[N-(2,6-dimethylphenyl)-N-(chloroacetyl)-amino]-propionate

Methoxyacetyl chloride
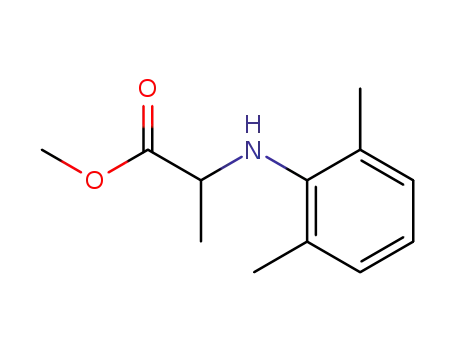
methyl N-(2,6-dimethylphenyl)alaninate
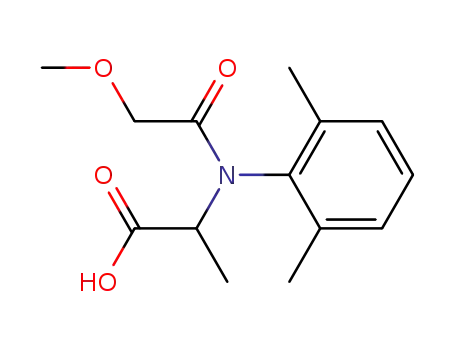
rac-2-[(2,6-dimethylphenyl)methoxyacetylamino]propionic acid
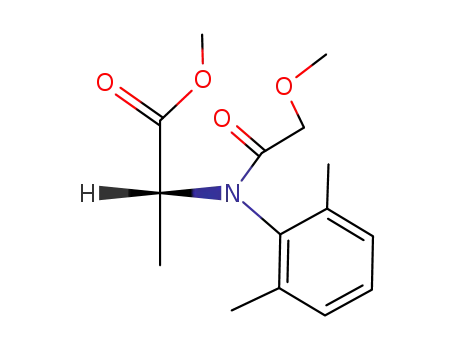
methyl N-(2,6-dimethylphenyl)-N-(methoxyacetyl)-D-alaninate
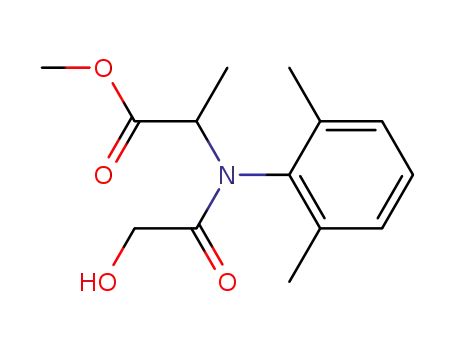
[N-(2,6-dimethylphenyl)-2-hydroxyacetamido]isopropanoate methyl ester
mercury(II)chloride*methyl N-(2,6-dimethylphenyl)-N-(2-methoxyacetyl)alaninate