Home > Products > intermediate
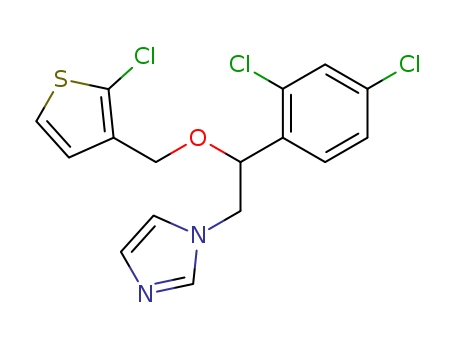







CasNo: 65899-73-2
MF: C16H13Cl3N2OS
|
Manufacturing Process |
A solution of 1-(2,4-dichlorophenyl)-2-(1-imidazolyl)ethanol (1.5 g, 5.8 mmol) dissolved in dry tetrahydrofuran (10 ml) was added to a stirred suspension of sodium hydride (0.39 g, as 80% dispersion in oil, 16 mmol) in dry tetrahydrofuran (10 ml) and warmed to 70°C for 90 minutes. The mixture was cooled in ice and a solution of 2-chloro-3- chloromethylthiophene (8.8 mmol) in dry tetrahydrofuran was added. The mixture was heated at 70°C for 3 hours and allowed to stir at room temperature overnight. The solvent was removed under vacuum and the residue stirred with dry ether (200 ml). The ether solution was filtered through Celite and saturated with hydrogen chloride gas to precipitate an oil which was solidified by trituration with ether and ethyl acetate. The solid product was collected and recrystallized from a mixture of acetone and diisopropyl ether to give the product, melting point 168°C to 170°C. |
|
Therapeutic Function |
Antifungal |
|
Definition |
ChEBI: A member of the class of imidazoles that comprises 2-(2,4-dichlorophenyl)ethylimidazole carrying an additional (2-chloro-3-thienyl)methoxy substituent at position 2. |
|
Brand name |
TROSYL |
InChI:InChI=1/C16H13Cl3N2OS/c17-12-1-2-13(14(18)7-12)15(8-21-5-4-20-10-21)22-9-11-3-6-23-16(11)19/h1-7,10,15H,8-9H2
Novel 1-aryl-2-(1-imidazolyl)alkyl ether...

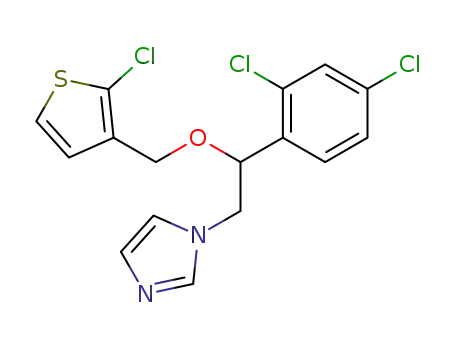
tioconazole
| Conditions | Yield |
|---|---|
|
|
|
|
|

tioconazole

C16H12(2)HCl3N2OS
| Conditions | Yield |
|---|---|
|
With
potassium carbonate; silver carbonate; johnphos;
In
dimethyl sulfoxide;
at 25 ℃;
for 12h;
|
85% |
|
With
water-d2; silver;
In
dimethyl sulfoxide;
at 40 ℃;
for 12h;
|
85% |
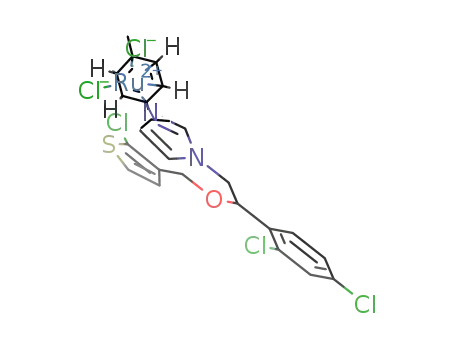
[(η6-p-cymene)RuCl2(tioconazole)]
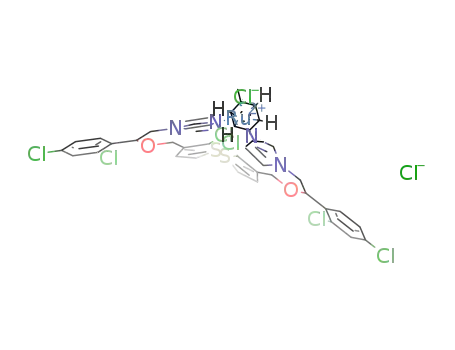
[(η6-p-cymene)RuCl(tioconazole)2]Cl