Home > Products > intermediate
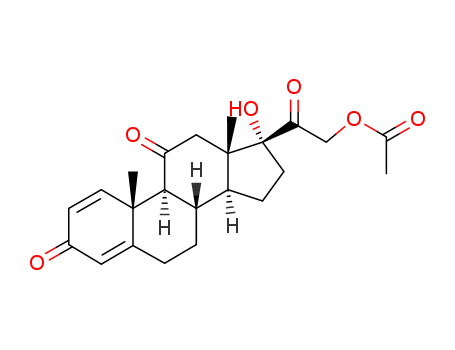







CasNo: 125-10-0
MF: C23H28O6
InChI:InChI=1/C23H28O6/c1-13(24)29-12-19(27)23(28)9-7-17-16-5-4-14-10-15(25)6-8-21(14,2)20(16)18(26)11-22(17,23)3/h6,8,10,16-17,20,28H,4-5,7,9,11-12H2,1-3H3/t16?,17?,20?,21?,22?,23-/m0/s1
-
The epoxidation of 1,4-dienyl 3-keto ste...
DMF dissolves o-iodoxybenzoic acid (up t...
The invention discloses a preparation me...
The invention discloses a 9-site dehalog...
The invention discloses a synthetic meth...
The stable pyridinium salt of o-iodoxybe...
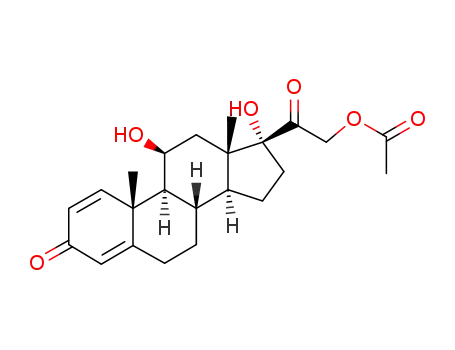
prednisolone 21-acetate

![(8S,9S,10R,11S,13S,14S)-11-Hydroxy-10,13-dimethyl-7,8,9,10,11,12,13,14,15,16-decahydro-6H-cyclopenta[a]phenanthrene-3,17-dione](/upload/2025/4/d170df3a-827a-46bd-a5ea-d995fa0c8f14.png)
(8S,9S,10R,11S,13S,14S)-11-Hydroxy-10,13-dimethyl-7,8,9,10,11,12,13,14,15,16-decahydro-6H-cyclopenta[a]phenanthrene-3,17-dione

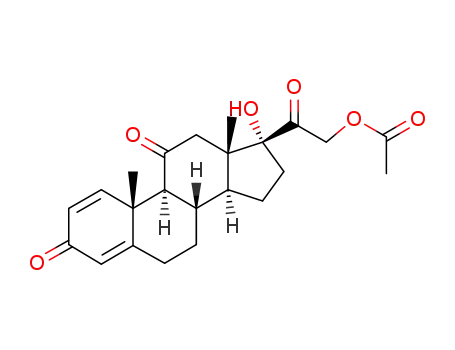
prednisone acetate
| Conditions | Yield |
|---|---|
|
Product distribution;
Rate constant;
Irradiation;
radiolytic degradation;
|

prednisolone 21-acetate


prednisone acetate
| Conditions | Yield |
|---|---|
|
With
1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione;
In
N,N-dimethyl-formamide;
at 23 - 25 ℃;
for 5h;
|
99% |
|
With
1-hydroxy-1.oxo-1H-1λ5-benzo[d][1,2]iodoxol-3-one pyridinium salt;
In
N,N-dimethyl-formamide;
at 24 - 28 ℃;
for 4h;
|
97% |
|
With
N-bromoacetamide;
|
|
|
With
chromium(VI) oxide;
|
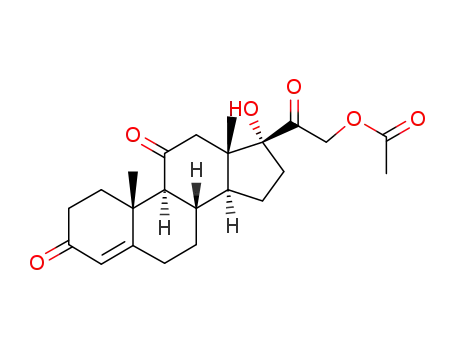
Cortisone acetate

prednisolone 21-acetate
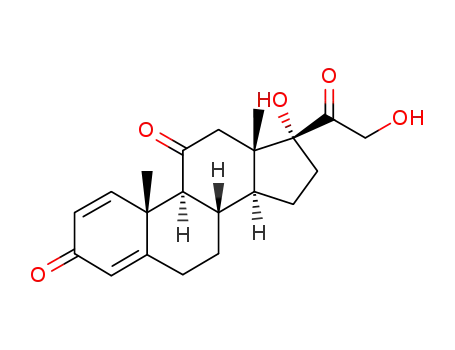
Prednison
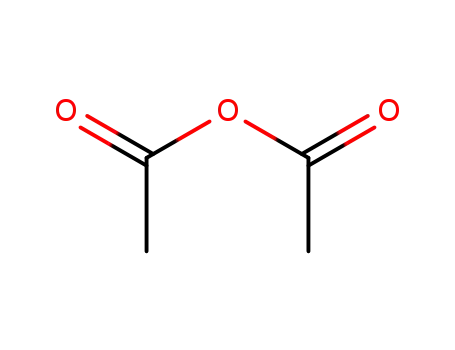
acetic anhydride
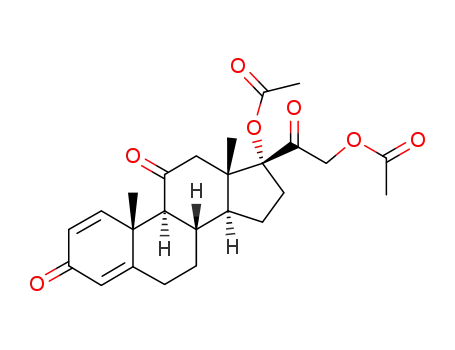
17α,21-diacetoxypregna-1,4-diene-3,11,20-trione
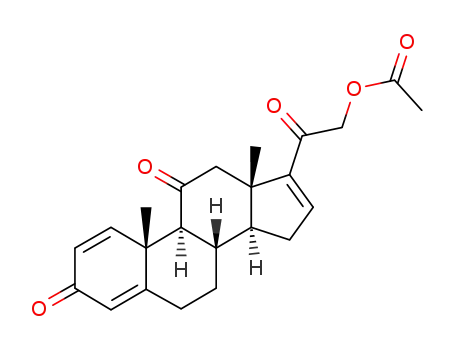
21-acetoxypregna-1,4,16-triene-3,11,20-trione

prednisolone 21-acetate
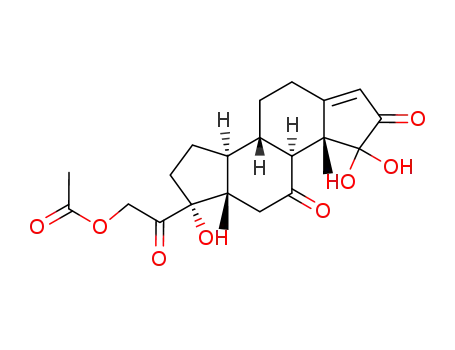
1α,1β,17α-Trihydroxy-21-acetoxy-A-nor-Δ3(5)-pregnentrion-(2,11,20)