Home > Products > intermediate
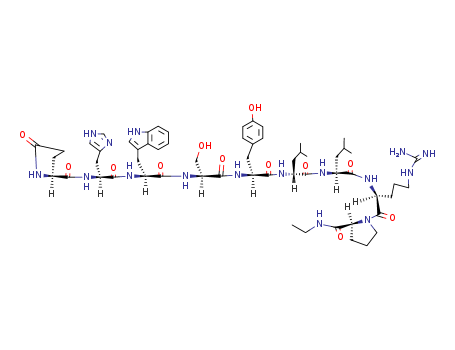







CasNo: 53714-56-0
MF: C59H84N16O12
Appearance: Fluffy solid.
|
Mechanism of action |
Leuprorelin is a nonapeptide synthetic analogue of Luteinizing Hormone Releasing Hormone (LHRH), which can promote the release of follicle stimulating hormone from the anterior pituitary, thereby reducing the increased testosterone concentration to castrate levels. When the administration is stopped, gonadotropins and androgens can return to normal concentrations. |
|
Pharmacokinetics |
Leuprolide acetate(Leuprorelin) is ineffective orally. Good absorption by subcutaneous or intramuscular injection. A single subcutaneous injection of 3.75mg, the blood concentration of 1 to 2 days peaked at 1 to 2ng/ml. For prostate cancer, 3.75mg is injected subcutaneously each time, once every 4 lookchem weeks, for a total of 3 injections, reaching a steady-state blood concentration of 0.1-1ng/ml. This product is hydrolyzed into 4 degradation products in the body and excreted by the kidneys. The urinary excretion rates of the original drug and metabolites were 2.9% and 1.5% after a single subcutaneous injection 28 days. |
|
Adverse reactions |
The main side effects of Leuprorelin are fever, heat sensation and elevated AST, ALT, γ-GTP and AKP. Sometimes there are facial flushing, sweating, loss of libido, impotence, feminized breasts, testicular atrophy, perineal discomfort and other endocrine system phenomena; abnormal electrocardiogram and increased ratio of heart and chest, bone pain, shoulder, lower back, limb pain, Urinary retention, frequent urination, hematuria, nausea, vomiting, loss of appetite, rash, itching and other allergic reactions and pain, induration, and redness at the injection site. Rarely, edema, chest pressure, chills, tiredness, weight gain, abnormal perception, tinnitus, hearing loss, TG, uric acid and BUN are increased. |
|
Indications |
Leuprolide is a potent LH-RH agonist for the first several days to a few weeks after initiation of therapy, and therefore, it initially stimulates testicular and ovarian steroidogenesis. Because of this initial stimulation of testosterone production, it is recommended that patients with prostatic cancer be treated concurrently with leuprolide and the antiandrogen flutamide (discussed earlier). Leuprolide is generally well tolerated, with hot flashes being the most common side effect. |
|
Therapeutic Function |
Antineoplastic |
|
Synthesis |
The synthesis process of Leuprorelin includes the following steps:(1) Fmoc-Pro-HMPB-AM resin is obtained from Fmoc-Pro-OH and HMPB-AM resin with a substitution degree of 0.2mmol/g~1.2mmol/g as starting materials;(2) The Fmoc-Pro-HMPB-AM resin was coupled one by one by Fmoc/tBu solid phase method to connect amino acids with protective groups in sequence, and the side chain fully protected leuprolide precursor peptide-HMPB-AM was synthesized Resin;(3) Cut the side chain fully protected leuprolide precursor peptide-HMPB-AM resin to obtain the side chain fully protected leuprolide precursor peptide;(4) Fully protected side chain leuprolide precursor peptide undergoes ethylamination to obtain side chain fully protected leuprolide;(5) Leuprolide is fully protected on the side chain by removing the side chain protecting group to obtain the crude leuprolide peptide;(6) The crude leuprorelin peptide is separated and purified by a high-pressure liquid phase column and lyophilized to obtain the leuprolide refined peptide. |
|
Definition |
ChEBI: An oligopeptide comprising pyroglutamyl, histidyl, tryptophyl, seryl, tyrosyl, D-leucyl, leucyl, arginyl, and N-ethylprolinamide residues joined in sequence. Leuprorelin is a synthetic nonapeptide analogue of gonadotropin-releasi g hormone, and is used as a subcutaneous hydrogel implant (particularly as the acetate salt) for the treatment of prostate cancer and for the suppression of gonadal sex hormone production in children with central precocious puberty. |
InChI:InChI=1/C59H84N16O12/c1-6-63-57(86)48-14-10-22-75(48)58(87)41(13-9-21-64-59(60)61)68-51(80)42(23-32(2)3)69-52(81)43(24-33(4)5)70-53(82)44(25-34-15-17-37(77)18-16-34)71-56(85)47(30-76)74-54(83)45(26-35-28-65-39-12-8-7-11-38(35)39)72-55(84)46(27-36-29-62-31-66-36)73-50(79)40-19-20-49(78)67-40/h7-8,11-12,15-18,28-29,31-33,40-48,65,76-77H,6,9-10,13-14,19-27,30H2,1-5H3,(H,62,66)(H,63,86)(H,67,78)(H,68,80)(H,69,81)(H,70,82)(H,71,85)(H,72,84)(H,73,79)(H,74,83)(H4,60,61,64)/t40-,41-,42-,43+,44-,45-,46-,47-,48-/m0/s1
It is demonstrated that gonadotropin-rel...
Post-translational modifications (PTMs) ...
Side-chain modifications that respond to...
A catalytic one-step synthesis of peptid...
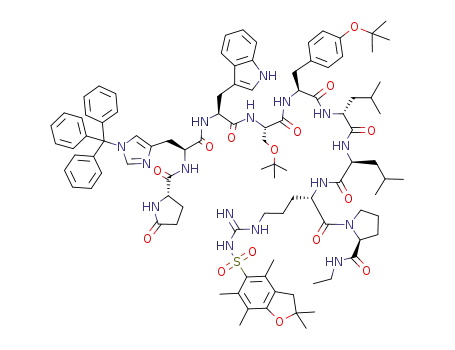
pGlu-His(Trt)-Trp-Ser(tBu)-Tyr(tBu)-D-Leu-Leu-Arg(Pbf)-Pro-NHEt

Leuprolide
| Conditions | Yield |
|---|---|
|
With
chlorotriisopropylsilane; ethane-1,2-dithiol; trifluoroacetic acid;
at 20 ℃;
for 2h;
|
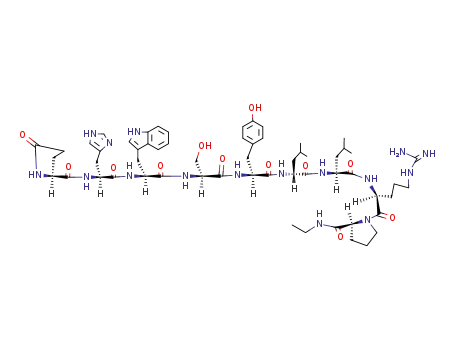
pGlu-His-Trp-Ser-Tyr-D-Leu-Leu-Arg(Mds)-Pro-NH-Et


Leuprolide
| Conditions | Yield |
|---|---|
|
With
methyl-phenyl-thioether; trifluoroacetic acid;
at 50 ℃;
for 1h;
Yield given;
|

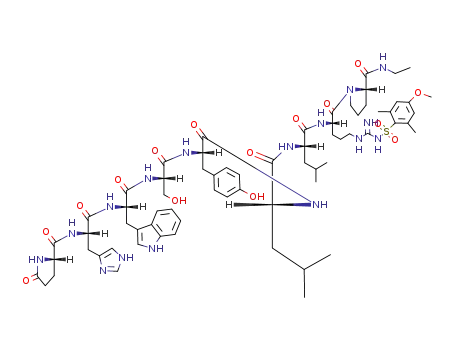
pGlu-His(Trt)-Trp-Ser(tBu)-Tyr(tBu)-D-Leu-Leu-Arg(Pbf)-Pro-NHEt