Home > Products > intermediate
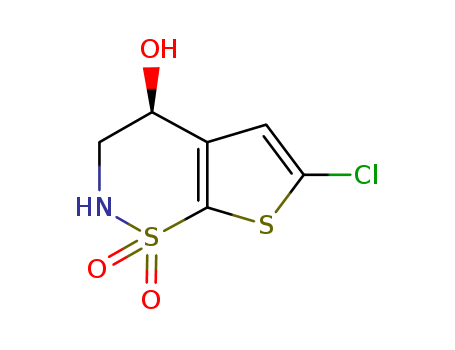







CasNo: 160982-16-1
MF: C6H6ClNO3S2
|
General Description |
(S)-6-Chloro-1,1-dioxo-1,2,3,4-tetrahydro-1lambda*6*-thieno[3,2-e][1,2]thiazin-4-ol is a chemical compound with the molecular formula C4H3ClNO3S2. It is a heterocyclic compound that contains both oxygen and sulfur atoms in its ring structure. (S)-6-CHLORO-1,1-DIOXO-1,2,3,4-TETRAHYDRO-1LAMBDA*6*-THIENO[3,2-E][1,2]THIAZIN-4-OL may have potential pharmaceutical applications due to its unique structure, and it could be used in the development of new drugs or as a reagent in chemical reactions. Further research and analysis are necessary to fully understand the properties and potential uses of this chemical compound. |
InChI:InChI=1/C6H6ClNO3S2/c7-5-1-3-4(9)2-8-13(10,11)6(3)12-5/h1,4,8-9H,2H2/t4-/m1/s1
A large scale synthesis of the topical c...
Sulfonamides and pharmaceutical composit...
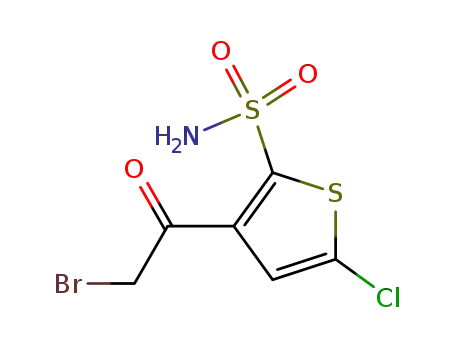
3-(2-Bromoacetyl)-5-chloro-thiophene-2-sulfonamide

![(S)-3,4-dihydro-6-chloro-4-hydroxy-4H-thieno[3,2-e]-1,2-thiazine-1,1-dioxide](/upload/2025/4/c8ca6b2f-bc56-47bd-a89e-c0f99da87584.png)
(S)-3,4-dihydro-6-chloro-4-hydroxy-4H-thieno[3,2-e]-1,2-thiazine-1,1-dioxide
| Conditions | Yield |
|---|---|
|
3-(2-Bromoacetyl)-5-chloro-thiophene-2-sulfonamide;
With
B-chlorodiisopinocampheylborane;
In
tert-butyl methyl ether;
at -40 - -32 ℃;
for 3.5h;
Inert atmosphere;
With
sodium hydroxide;
In
tert-butyl methyl ether; water;
at 0 - 22 ℃;
for 2h;
enantioselective reaction;
|
77% |

3-(2-Bromoacetyl)-5-chloro-thiophene-2-sulfonamide

(+)-β-chlorodiisopino-campheylborane

tert-butyl methyl ether

carbon dioxide

![(S)-3,4-dihydro-6-chloro-4-hydroxy-4H-thieno[3,2-e]-1,2-thiazine-1,1-dioxide](/upload/2025/4/c8ca6b2f-bc56-47bd-a89e-c0f99da87584.png)
(S)-3,4-dihydro-6-chloro-4-hydroxy-4H-thieno[3,2-e]-1,2-thiazine-1,1-dioxide
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; sodium hydroxide;
In
toluene;
|

3-(2-Bromoacetyl)-5-chloro-thiophene-2-sulfonamide

tert-butyl methyl ether

carbon dioxide
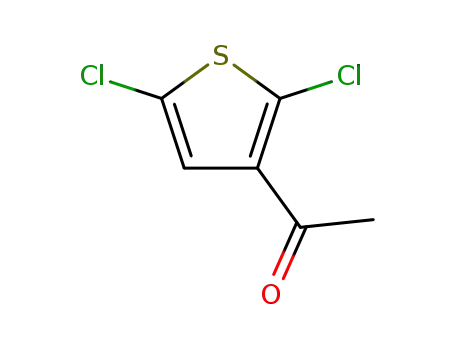
3-acetyl-2,5-dichlorothiophene
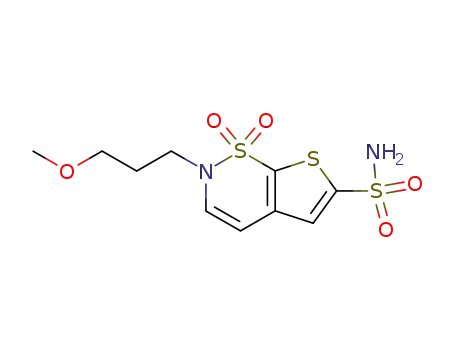
2-(3-methoxypropyl)-2H-thieno[3,2-e]-1,2-thiazine-6-sulfonamine 1,1-dioxide
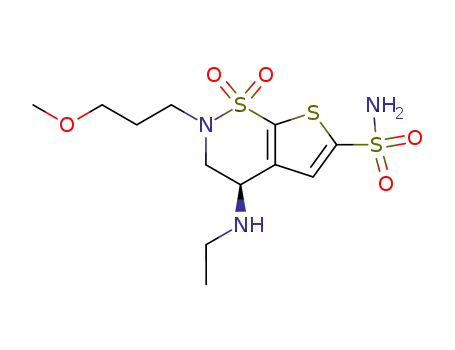
brinzolamide
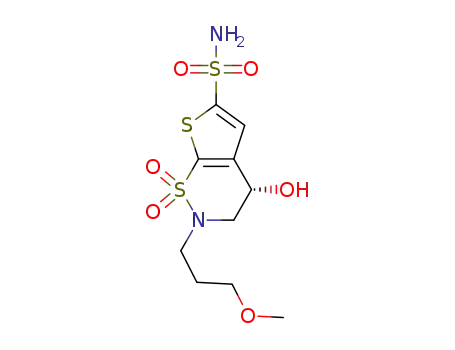
(S)-3,4-dihydro-4-hydroxy-2(3-methoxypropyl)-4H-thieno[3,2-e]-1,2-thiazine-6-sulfonamide-1,1-dioxide
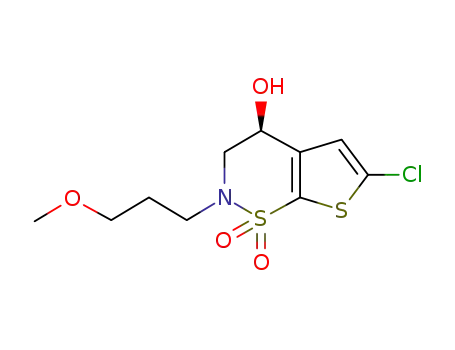
(S)-3,4-dihydro-6-chloro-4-hydroxy-2-(3-methoxypropyl)-4H-thieno[3,2-e]-1,2-thiazine 1,1-dioxide