Home > Products > intermediate
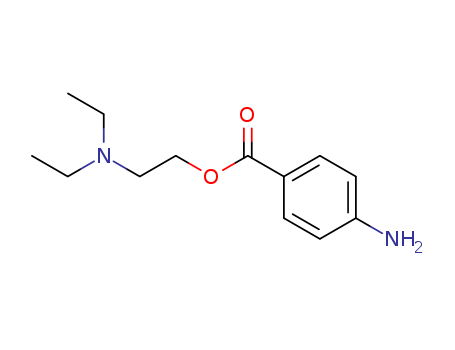







CasNo: 59-46-1
MF: C13H20N2O2
|
Biological Functions |
Procaine hydrochloride (Novocain) is readily hydrolyzed by plasma cholinesterase, although hepatic metabolism also occurs. It is not effective topically but is employed for infiltration, nerve block, and spinal anesthesia. It has a relatively slow onset and short (1 hour) duration of action. All concentrations can be combined with epinephrine. It is available in dental cartridges with phenylephrine as the vasoconstrictor. |
|
Purification Methods |
Procain crystallises as the dihydrate from aqueous EtOH and as the anhydrous material from pet ether or diethyl ether. The latter is hygroscopic. [Beilstein 14 IV 1138.] |
|
Definition |
ChEBI: A benzoate ester, formally the result of esterification of 4-aminobenzoic acid with 2-diethylaminoethanol but formed experimentally by reaction of ethyl 4-aminobenzoate with 2-diethylaminoethanol. |
|
General Description |
Procaine was synthesized in 1904 to address the chemical instabilityof cocaine and the local irritation it produced. The pKa of procaine is 8.9; it has low lipid solubility and the estergroup is unstable in basic solutions. Procaine is available inconcentrations ranging from 0.25% to 10% with pHs adjustedto 5.5 to 6.0 for chemical stability. Procaine is also includedin some formulations of penicillin G to decrease the pain ofintramuscular injection. |
InChI:InChI=1/C13H20N2O2/c1-3-15(4-2)9-10-17-13(16)11-5-7-12(14)8-6-11/h5-8H,3-4,9-10,14H2,1-2H3
-
Pt-nanoparticles supported on halloysite...
The present disclosure provides ammonium...
Augmenting the modified naturally occurr...
Efficient and selective aerobic oxidatio...
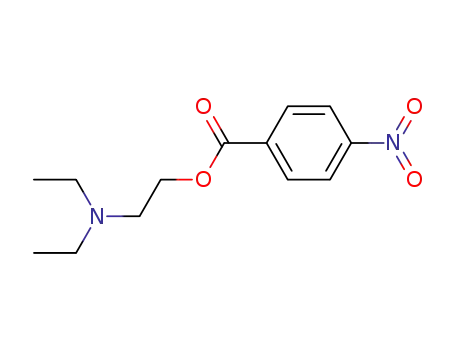
2-(diethylamino)ethyl 4-nitrobenzoate

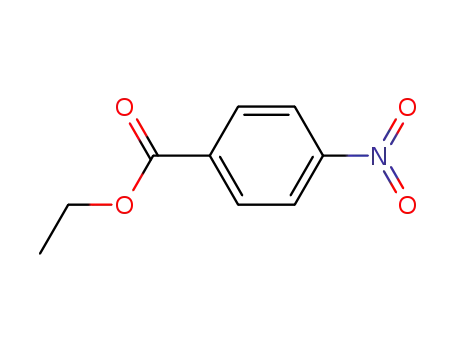
ethyl 4-nitrobenzoate

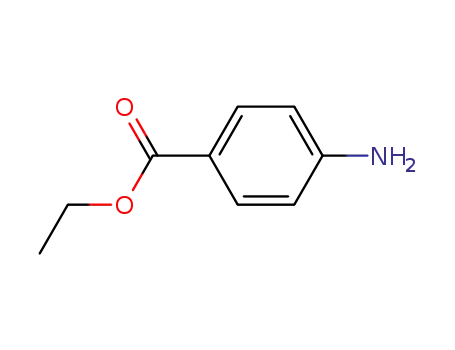
p-aminoethylbenzoate

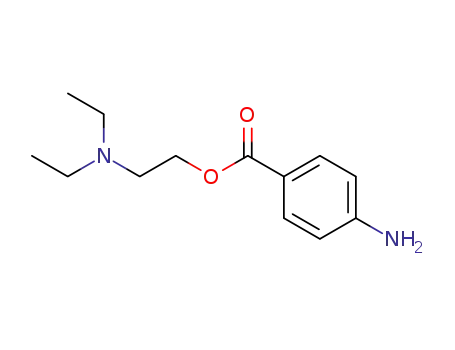
Procaine
| Conditions | Yield |
|---|---|
|
With
hydrogen;
In
ethanol;
at 24.84 ℃;
for 3h;
under 750.075 Torr;
chemoselective reaction;
|

2-(diethylamino)ethyl 4-nitrobenzoate

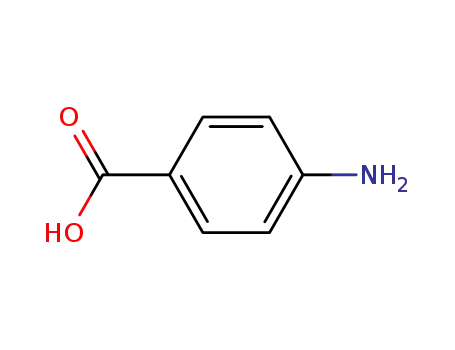
4-amino-benzoic acid


Procaine

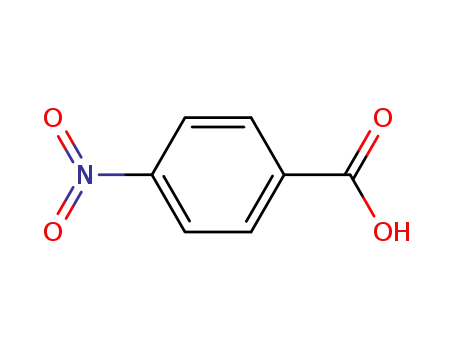
4-nitro-benzoic acid
| Conditions | Yield |
|---|---|
|
With
hydrogen;
In
ethyl acetate;
at 24.84 ℃;
for 24h;
under 750.075 Torr;
chemoselective reaction;
|

2-(diethylamino)ethyl 4-nitrobenzoate
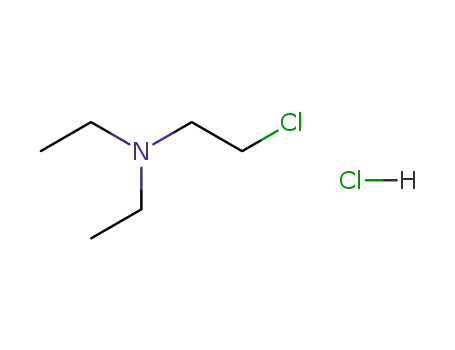
2-chloro-N,N-diethylethylamine hydrochloride

4-amino-benzoic acid
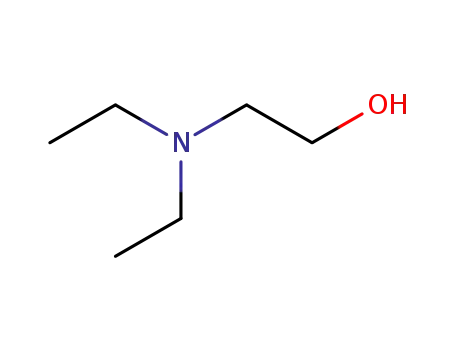
2-(Diethylamino)ethanol
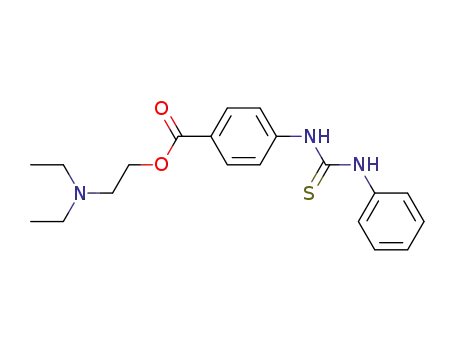
4-(N'-phenyl-thioureido)-benzoic acid-(2-diethylamino-ethyl ester)
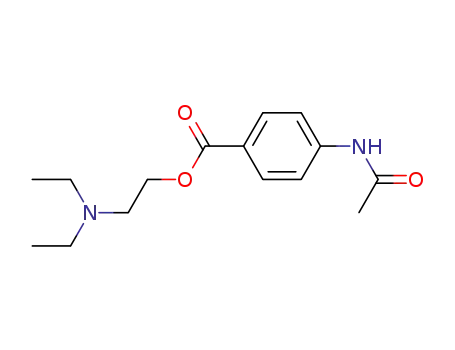
2-(diethylaminoethyl) 4-acetylaminobenzoate
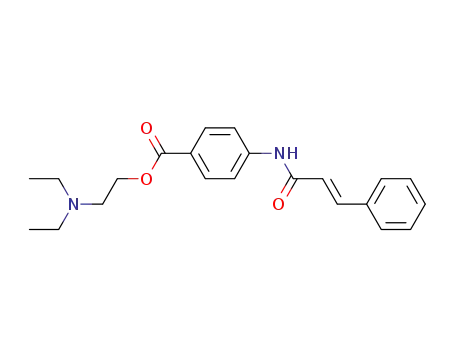
4-trans-cinnamoylamino-benzoic acid-(2-diethylamino-ethyl ester)
4-Thioureido-benzoic acid 2-diethylamino-ethyl ester