Home > Products > intermediate
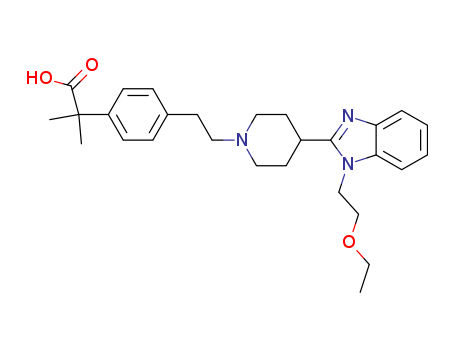







CasNo: 202189-78-4
MF: C28H37 N3 O3
|
Synthesis |
In 2011, Collier and co-workers published a communication describing both the original synthesis of bilastine and an improved route which was amenable to gram-scale production. Collier’s second generation route, shown below, relies upon a convergent approach involving the union of piperidinyl benzimidazole 49 with fully functionalized phenethyl electrophile 48.Coupling the commercially available bromophenyl acetate 44 with cyclic trioxatriborinane 45 under conventional Suzuki conditions furnished styrene 46 in good yield. Alternatively, this vinylation reaction was also performed under Stille conditions with tributyl vinyl stannane in 83% yield. Hydroboration–oxidation of 46 delivered phenethyl alcohol 47 which was then immediately mesylated under basic conditions in toluene to produce adduct 48. This sulfonate was then reacted with piperidine 49 (whose preparation is described in Scheme 7) followed by saponification of the resulting ester 50 to arrive at bilastene (VI) in 26% overall yield from 44. For the preparation of bilastine piperidine 49, commercially available piperidine 51 was first protected as the Boc-carbamate 52 prior to alkylation of the benzimidazole nitrogen atom with 1-chloro-2-ethoxyethane 53, providing compound 54. The Boc group of 54 was removed under acidic conditions to give fragment 49. This sequence produced the desired piperidine component in 86% overall yield from 51. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antivirals: concentration possibly increased by ritonavir. Grapefruit juice: concentration of bilastine reduced. |
|
Metabolism |
Not significantly metabolised. Almost 95% of the administered dose was recovered in urine (28.3%) and faeces (66.5%) as unchanged bilastine |
|
Brand name |
Bilaxten |
InChI:InChI=1/C28H37N3O3/c1-4-34-20-19-31-25-8-6-5-7-24(25)29-26(31)22-14-17-30(18-15-22)16-13-21-9-11-23(12-10-21)28(2,3)27(32)33/h5-12,22H,4,13-20H2,1-3H3,(H,32,33)
The invention belongs to the technical f...
The present invention relates to a proce...
The invention provides a preparation met...
The invention belongs to the technical f...
![2-(2-(4-(2-(4-(1-(2-ethoxyethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)propan-2-yl)-4,4-dimethyl-4,5-dihydrooxazole](/upload/2025/4/ec96ce99-8365-4e23-9f7f-f504923a98d4.png)
2-(2-(4-(2-(4-(1-(2-ethoxyethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)propan-2-yl)-4,4-dimethyl-4,5-dihydrooxazole

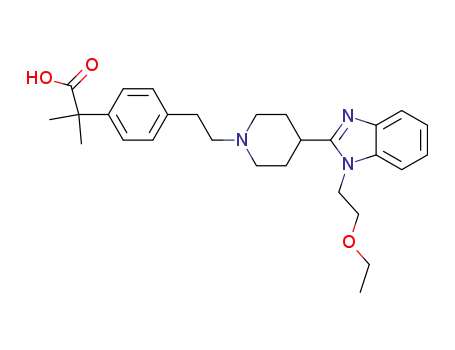
Bilastine
| Conditions | Yield |
|---|---|
|
With
potassium hypochlorite; tetra(n-butyl)ammonium hydrogensulfate;
In
water; ethyl acetate;
at 20 ℃;
for 12h;
Reagent/catalyst;
Temperature;
|
93% |
|
With
succinic acid;
for 36h;
Reflux;
|
93.5% |
|
2-(2-(4-(2-(4-(1-(2-ethoxyethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)propan-2-yl)-4,4-dimethyl-4,5-dihydrooxazole;
With
hydrogenchloride; water;
Reflux;
With
sodium hydroxide;
In
water;
pH=7;
|
87% |
|
With
hydrogenchloride; water;
Reflux;
|
87% |
|
2-(2-(4-(2-(4-(1-(2-ethoxyethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)propan-2-yl)-4,4-dimethyl-4,5-dihydrooxazole;
With
acetic acid;
In
water;
for 3h;
Reflux;
With
water; sodium hydroxide;
Reagent/catalyst;
Reflux;
|
85% |
|
With
hydrogenchloride;
In
water;
Solvent;
Reflux;
|
![4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α-dimethylphenylacetic acid methyl ester](/upload/2025/4/cd6cb4d1-7953-4a9a-ac75-6917eb4258eb.png)
4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α-dimethylphenylacetic acid methyl ester


Bilastine
| Conditions | Yield |
|---|---|
|
With
water; sodium hydroxide;
In
ethanol;
for 16h;
Reflux;
|
96% |
|
With
sodium hydroxide;
In
ethanol; water;
for 7h;
Reflux;
|
96% |
|
With
methanol; sodium hydroxide;
for 5h;
Reflux;
|
96% |
|
With
lithium hydroxide;
In
methanol; water;
at 20 ℃;
for 6h;
Reagent/catalyst;
|
92.1% |
|
With
methanol; sodium hydroxide;
at 0 - 20 ℃;
for 3h;
|
91.3% |
|
4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α-dimethylphenylacetic acid methyl ester;
With
sodium hydroxide;
In
ethanol;
at 50 - 55 ℃;
for 3h;
With
acetic acid;
In
ethanol; water;
pH=7;
|
90% |
|
With
water; sodium hydroxide;
In
ethanol;
for 1h;
Reflux;
|
89.3% |
|
4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α-dimethylphenylacetic acid methyl ester;
With
water; sodium hydroxide;
In
ethanol;
for 3h;
Reflux;
With
hydrogenchloride;
In
water;
at 20 ℃;
pH=5-6;
Solvent;
|
82.2% |
|
4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α-dimethylphenylacetic acid methyl ester;
With
water; lithium hydroxide;
In
tetrahydrofuran; methanol;
at 70 ℃;
for 16h;
With
hydrogenchloride;
In
tetrahydrofuran; methanol; water;
|
64% |
|
Multi-step reaction with 2 steps
1: hydrogenchloride / diethyl ether / 0 - 5 °C
2: water; potassium hydroxide / methanol / 16 h / 100 °C / Inert atmosphere
With
hydrogenchloride; water; potassium hydroxide;
In
methanol; diethyl ether;
|
|
|
With
sodium hydroxide;
In
ethanol;
at 65 - 70 ℃;
for 4h;
|
7 g |
|
With
lithium hydroxide;
In
tetrahydrofuran;
|
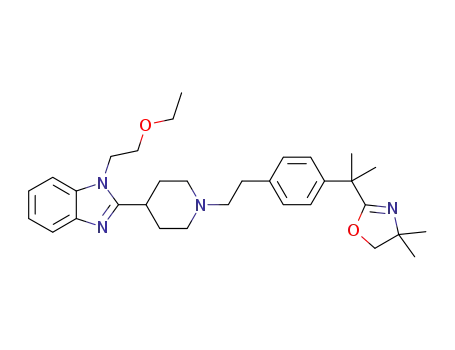
2-(2-(4-(2-(4-(1-(2-ethoxyethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)propan-2-yl)-4,4-dimethyl-4,5-dihydrooxazole
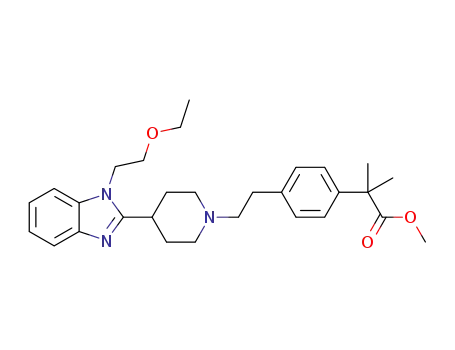
4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α-dimethylphenylacetic acid methyl ester
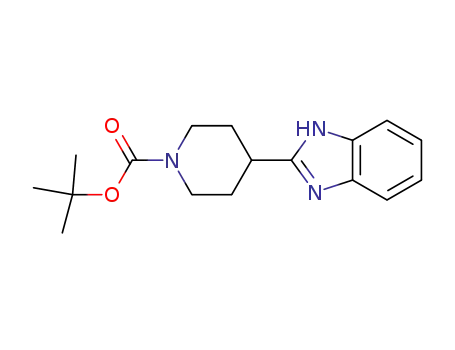
tert-butyl 4-(1H-benzo[d]imidazol-2-yl)piperidine-1-carboxylate
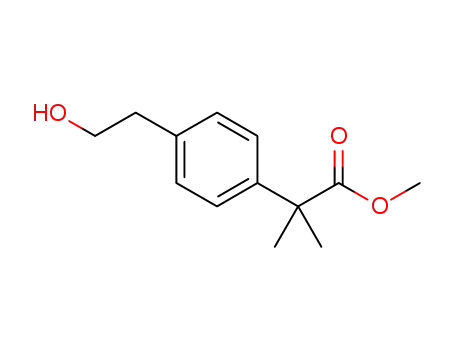
2-methyl-2-(4-(2-hydroxyethyl)phenyl)propionic acid methyl ester