Home > Products > intermediate







CasNo: 518048-03-8
MF: C16H19FN4O3
Appearance: White solid (powder)
InChI:InChI=1/C16H19FN4O3/c1-16(2,18)15-20-11(12(22)14(24)21(15)3)13(23)19-8-9-4-6-10(17)7-5-9/h4-7,22H,8,18H2,1-3H3,(H,19,23)
A facile, cost-effective, and commercial...
Raltegravir sodium synthesis was achieve...
The invention discloses a pyrimidinone a...
The present work describes a two-step pr...
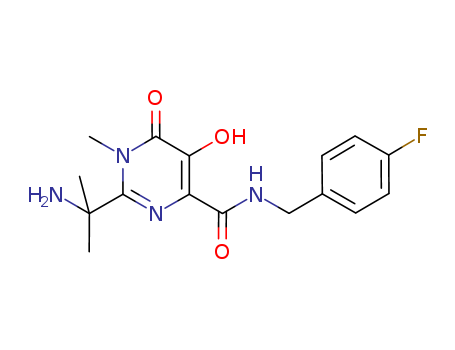
![{1-[4-(4-fluoro-benzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydro-pyrimidin-2-yl]-1-methyl-ethyl}carbamic acid benzyl ester](/upload/2025/4/9613aee2-2fe3-48d5-9808-7f792a46f217.png)
{1-[4-(4-fluoro-benzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydro-pyrimidin-2-yl]-1-methyl-ethyl}carbamic acid benzyl ester

![2-(1-amino-1-methyl-ethyl)-N-[(4-fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-6-oxo-4-pyrimidinecarboxamide](/upload/2025/4/b08bff5c-2e22-4cb5-a979-ca6ef0778c48.png)
2-(1-amino-1-methyl-ethyl)-N-[(4-fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-6-oxo-4-pyrimidinecarboxamide
| Conditions | Yield |
|---|---|
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
|
100% |
|
{1-[4-(4-fluoro-benzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydro-pyrimidin-2-yl]-1-methyl-ethyl}carbamic acid benzyl ester;
With
methanesulfonic acid; 5%-palladium/activated carbon; hydrogen;
In
methanol;
at 12 ℃;
under 775.743 Torr;
Large scale reaction;
With
sodium hydroxide;
In
methanol; water;
at 5 - 23 ℃;
pH=8;
|
99% |
|
{1-[4-(4-fluoro-benzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydro-pyrimidin-2-yl]-1-methyl-ethyl}carbamic acid benzyl ester;
With
formic acid; potassium formate;
palladium 10% on activated carbon;
In
methanol; water;
at 50 ℃;
for 2.5h;
With
sodium hydroxide;
In
methanol; water;
at 5 - 20 ℃;
for 21h;
|
98% |
|
With
methanesulfonic acid; hydrogen;
5%-palladium/activated carbon;
In
methanol;
at 20 - 50 ℃;
for 3 - 4h;
under 2828.7 Torr;
|
96% |
|
With
methanesulfonic acid; hydrogen;
5% palladium over charcoal;
In
methanol;
at 20 - 50 ℃;
for 3 - 4h;
under 2828.7 Torr;
|
96% |
|
With
methanesulfonic acid; hydrogen;
5% palladium over charcoal;
In
methanol;
at 50 ℃;
for 3 - 4h;
under 2068.65 Torr;
|
96% |
|
With
methanesulfonic acid; hydrogen;
5% palladium over charcoal;
In
methanol;
at 20 - 50 ℃;
for 3 - 4h;
under 2828.7 Torr;
|
96% |
|
{1-[4-(4-fluoro-benzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydro-pyrimidin-2-yl]-1-methyl-ethyl}carbamic acid benzyl ester;
With
methanesulfonic acid; water; hydrogen;
5% palladium over charcoal;
In
methanol;
at 50 ℃;
for 3 - 4h;
under 2828.7 Torr;
With
sodium hydroxide;
In
methanol; water;
pH=7 - 8.0;
|
96% |
|
With
5%-palladium/activated carbon; hydrogen;
In
methanol;
at 25 ℃;
under 6723.1 Torr;
|
91% |
|
With
hydrogen bromide;
In
water;
at 28 - 62 ℃;
for 2.5h;
|
|
|
With
glycolic Acid; 5%-palladium/activated carbon; hydrogen;
In
methanol;
under 6723.1 Torr;
Reagent/catalyst;
|
65 g |
|
With
sodium hydroxide;
In
ethanol; butan-1-ol;
Reagent/catalyst;
Reflux;
|
3 g |
![N-[(4-fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(methoxy)carbonyl]amino]ethyl]-6-oxo-4-pyrimidine carboxamide](/upload/2025/4/7e4b30de-1279-4560-b04f-5fe644654bc0.png)
N-[(4-fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(methoxy)carbonyl]amino]ethyl]-6-oxo-4-pyrimidine carboxamide

![2-(1-amino-1-methyl-ethyl)-N-[(4-fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-6-oxo-4-pyrimidinecarboxamide](/upload/2025/4/b08bff5c-2e22-4cb5-a979-ca6ef0778c48.png)
2-(1-amino-1-methyl-ethyl)-N-[(4-fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-6-oxo-4-pyrimidinecarboxamide
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
butan-1-ol;
at 105 - 110 ℃;
for 3h;
Concentration;
Solvent;
Temperature;
Time;
|
85% |
|
With
sodium hydroxide;
In
butan-1-ol;
at 100 - 110 ℃;
for 2h;
Concentration;
Temperature;
Reagent/catalyst;
Solvent;
|
79% |
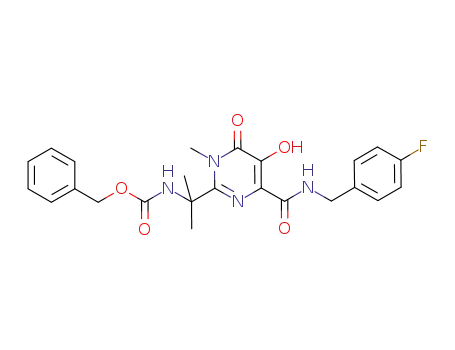
{1-[4-(4-fluoro-benzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydro-pyrimidin-2-yl]-1-methyl-ethyl}carbamic acid benzyl ester
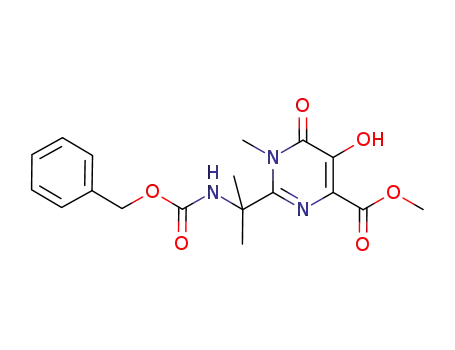
2-(1-benzyloxycarbonylamino-1-methyl-ethyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydro-pyrimidine-4-carboxylic acid methyl ester
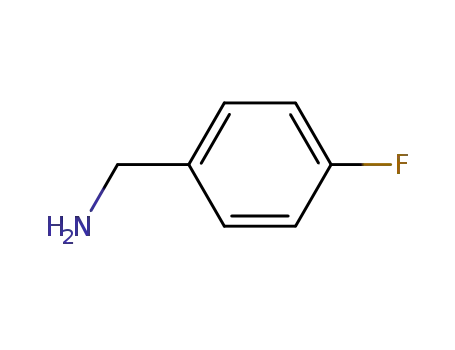
para-fluorobenzylamine
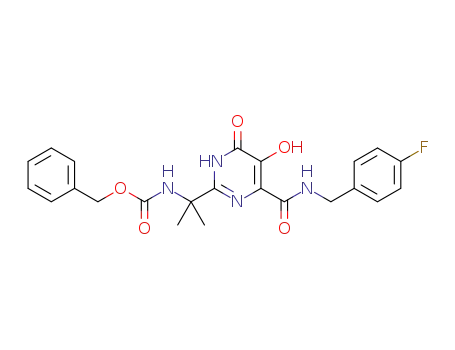
benzyl 2-(4-(4-fluorobenzylcarbamoyl)-5-hydroxy-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-ylcarbamate
raltegravir
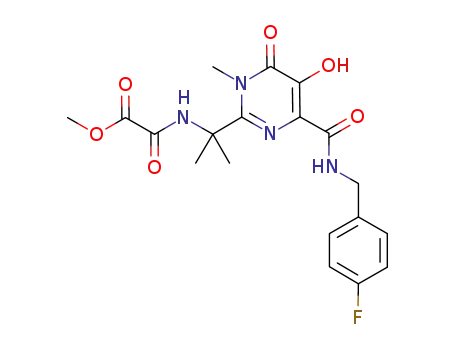
methyl {[1-(4-{[(4-fluorobenzyl)amino]carbonyl}-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)-1-methylethyl]amino}(oxo)acetate
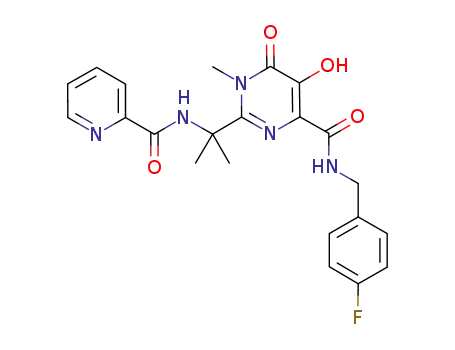
N-(4-fluorobenzyl)-5-hydroxy-1-methyl-2-(1-methyl-1-[(pyridin-2ylcarbonyl)amino]ethyl)-6-oxo-1,6-dihydropyrimidine-4-carboxamide
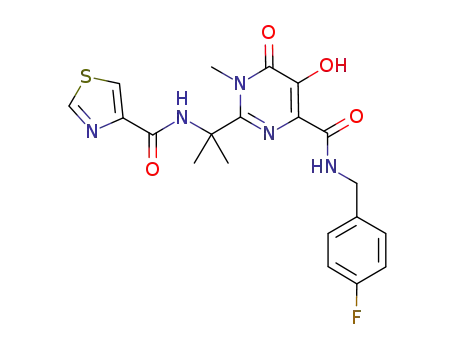
N-(4-fluorobenzyl)-5-hydroxy-1-methyl-2-{1-methyl-1-[(1,3-thiazol-5-ylcarbonyl)amino]ethyl}-6-oxo-1,6-dihydropyrimidine-4-carboxamide