Home > Products > intermediate







CasNo: 76855-69-1
MF: C13H25NO4Si
Appearance: white to light yellow crystal powder
|
Purification Methods |
Purify it by chromatography on silica gel (3 x 14cm) for 50g of ester using 20% EtOAc in n-hexane. The eluate is evaporated, and the residue is recrystallised from hexane (white fluffy crystals). [Leanza et al. Tetrahedron 39 2505 1983.] |
InChI:InChI=1/C13H25NO4Si/c1-8(18-19(6,7)13(3,4)5)10-11(16)14-12(10)17-9(2)15/h8,10,12H,1-7H3,(H,14,16)/t8-,10+,12-/m1/s1
The invention discloses a preparation me...
The invention provides a method for remo...
The invention provides a preparation met...
The invention discloses a preparation me...
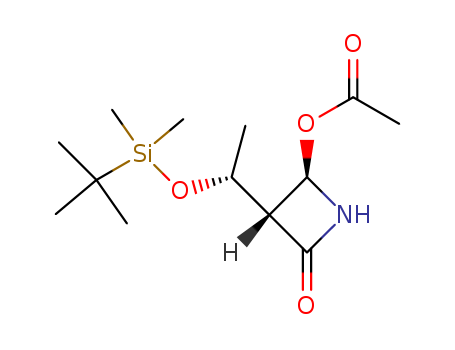
<3S-<3α(S*),4β>>-3-(1-t-butyldimethylsilyloxyethyl)-4-acetyl-2-azetidinone

![(3R,4R)-3-[(R)-1-(tert-butyldimethylsilyloxy)ethyl]-4-acetoxyazetidin-2-one](/upload/2025/4/4c5c358a-42d5-4342-a61a-543cd645e2ec.png)
(3R,4R)-3-[(R)-1-(tert-butyldimethylsilyloxy)ethyl]-4-acetoxyazetidin-2-one
| Conditions | Yield |
|---|---|
|
With
3-chloro-benzenecarboperoxoic acid;
In
ethyl acetate;
|
93% |
|
With
3-chloro-benzenecarboperoxoic acid;
In
ethyl acetate;
at 35 ℃;
for 2h;
|
93% |
|
<3S-<3α(S*),4β>>-3-(1-t-butyldimethylsilyloxyethyl)-4-acetyl-2-azetidinone;
With
sodium perborate; acetic acid;
at 60 ℃;
for 2h;
With
sodium acetate;
In
acetic acid;
at 60 ℃;
for 2h;
|
92% |
|
With
3-chloro-benzenecarboperoxoic acid;
In
chloroform;
at 25 ℃;
for 18h;
|
84% |
|
With
3-chloro-benzenecarboperoxoic acid;
In
chloroform;
for 96h;
Ambient temperature;
|
84% |
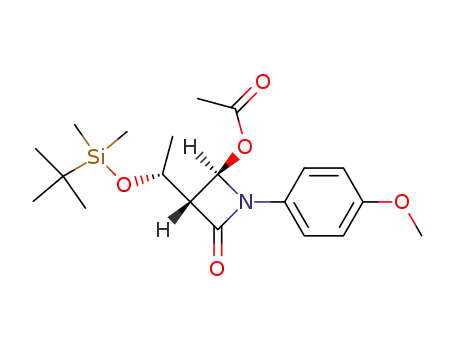
(2R,3R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-1-(4-methoxyphenyl)-4-oxoazetidin-2-yl acetate

![(3R,4R)-3-[(R)-1-(tert-butyldimethylsilyloxy)ethyl]-4-acetoxyazetidin-2-one](/upload/2025/4/4c5c358a-42d5-4342-a61a-543cd645e2ec.png)
(3R,4R)-3-[(R)-1-(tert-butyldimethylsilyloxy)ethyl]-4-acetoxyazetidin-2-one
| Conditions | Yield |
|---|---|
|
(2R,3R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-1-(4-methoxyphenyl)-4-oxoazetidin-2-yl acetate;
With
ozone;
In
methanol;
at -20 ℃;
With
sodium thiosulfate;
In
methanol; water;
at 8 ℃;
for 1h;
With
thiourea;
In
methanol; water;
at 40 ℃;
|
95% |
|
(2R,3R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-1-(4-methoxyphenyl)-4-oxoazetidin-2-yl acetate;
With
sodium acetate; ozone;
In
methanol;
at -14 ℃;
With
carbon monoxide;
In
methanol;
at 30 ℃;
for 2h;
under 11251.1 Torr;
Temperature;
Pressure;
Autoclave;
|
85.2% |
|
With
ammonium cerium(IV) nitrate;
In
water; acetonitrile;
at -10 ℃;
for 0.2h;
|
83% |
|
With
sodium acetate; ozone; sodium hydrogensulfite;
In
methanol;
at -14 - 35 ℃;
for 0.5h;
|
81% |
|
(2R,3R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-1-(4-methoxyphenyl)-4-oxoazetidin-2-yl acetate;
With
ozone;
In
methanol;
at -22 - -10 ℃;
for 6h;
With
sodium thiosulfate;
In
methanol;
at 8 ℃;
for 1h;
With
thiourea;
In
methanol;
at 40 ℃;
for 3h;
Temperature;
|
69.7% |
|
With
ozone;
In
methanol;
at -20 ℃;
for 3h;
Product distribution / selectivity;
|
|
|
With
water; lithium perchlorate;
In
acetonitrile;
Product distribution / selectivity;
Electrolysis;
|
|
|
With
ammonium cerium(IV) nitrate; water;
at -15 ℃;
for 0.5h;
Product distribution / selectivity;
|
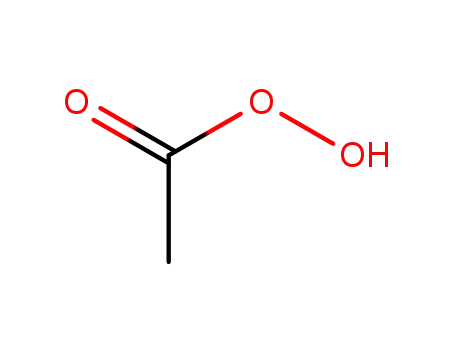
peracetic acid
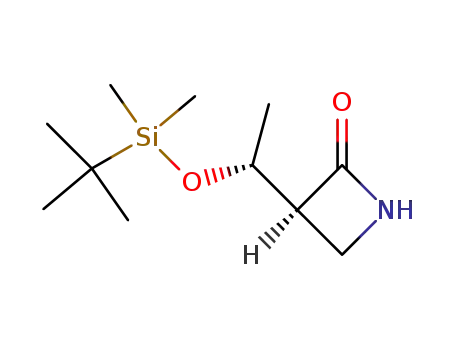
(1'R,3S)-3-<1'-<(tert-butyldimethylsilyl)oxy>ethyl>azetidin-2-one
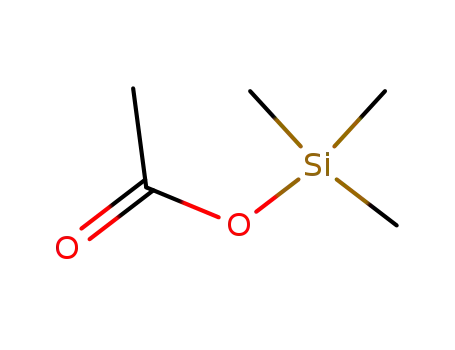
trimethylsilyl acetate
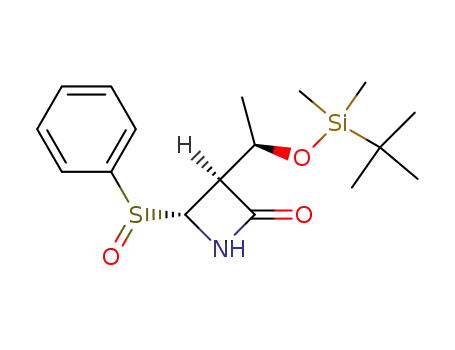
(3S,4R)-3-<(1R)-1-(tert-Butyldimethylsiloxy)ethyl>-4-phenylsulfinylazetidin-2-one
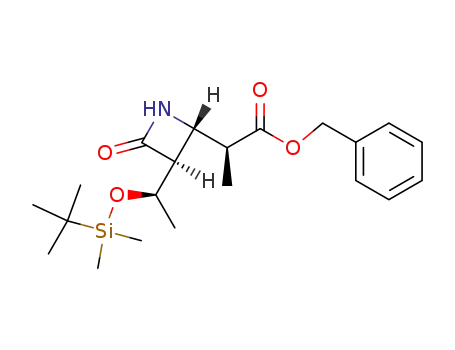
(3S,4S)-4-<(1S)-1-benzyloxycarbonylethyl>-3-<(1R)-1-tert-butyldimethylsilyloxyethyl>-2-azetidinone
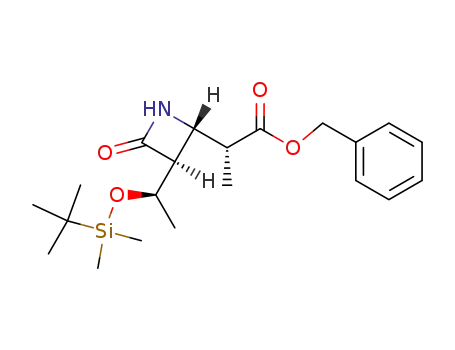
(3S,4S)-4-<(1R)-1-benzyloxycarbonylethyl>-3-<(1R)-1-tert-butyldimethylsilyloxyethyl>-2-azetidinone
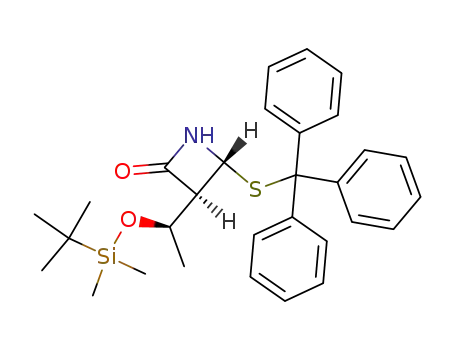
(3S,4R)-3-((R)-1-(t-butyldimethylsilyloxy)ethyl)-4-triphenylmethylthio-2-azetidinone
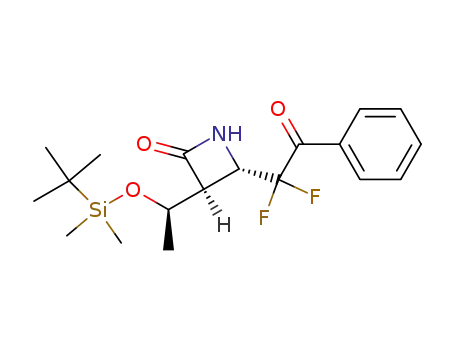
(3R,4R)-3-(1R-t-butylsilyloxyethyl)-4-(2-phenyl-2-oxo-1,1-difluoro)-azetidin-2-one